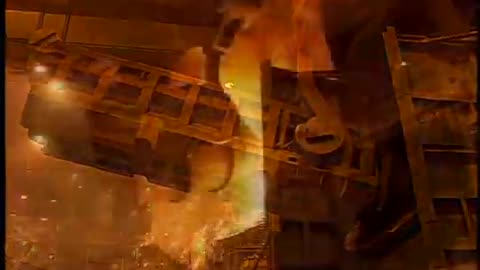Premium Only Content
The Steel Making Process From Start to Finish - Material Science
RCast iron pan manufacturing process
RMaking a Caterpillar Engine Block at the Mapleton, IL Foundry
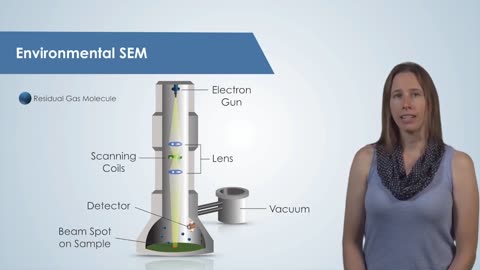
RIntroduction to the Scanning Electron Microscope (SEM)
Magnetic Particle Inspection: Material Science
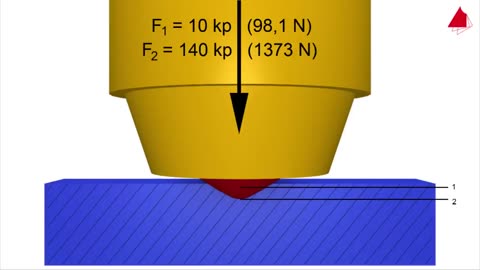
Vickers Hardness Test - Material Science
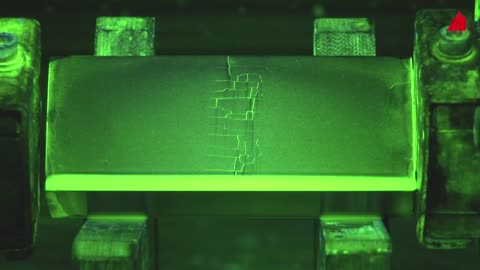
Metallography - Macroscopic Techniques - Material Science
Dye Penetrant Inspection - Material Science
Brinell Hardness Test - Material Science
Rockwell Hardness Test: Material Science
Fatigue Test - Material Science
Material Science: Eddy Current Testing
Tensile Test: Material Science
The Scanning Electron Microscope: Material Science
Microscopic Techniques - Metallography - Material Science
Material Science: X-ray Inspection and Industrial Computed Tomography
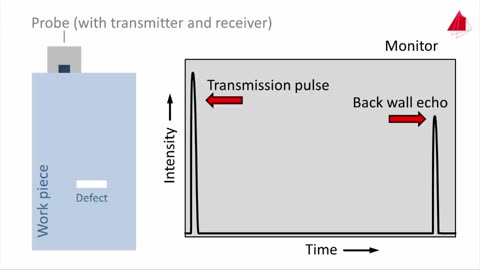
Ultrasonic Testing Basics: Material Science NDT
The Steel Making Process From Start to Finish - Material Science
The Steel Making Process From Start to Finish
Steel is an alloy of iron and carbon with improved strength and fracture resistance compared to other forms of iron. Because of its high tensile strength and low cost, steel is one of the most commonly manufactured materials in the world. Steel is used in buildings, as concrete reinforcing rods, in bridges, infrastructure, tools, ships, trains, cars, bicycles, machines, electrical appliances, furniture, and weapons.
Iron is always the main element in steel, but many other elements may be present or added. Stainless steels, which are resistant to corrosion and oxidation, typically need an additional 11% chromium.
Steel is primarily produced using one of two methods: Blast Furnace or Electric Arc Furnace.
The blast furnace is the first step in producing steel from iron oxides. The first blast furnaces appeared in the 14th century and produced one ton per day. Even though equipment is improved and higher production rates can be achieved, the processes inside the blast furnace remain the same. The blast furnace uses coke, iron ore and limestone to produce pig iron.
Coal traditionally has been a key part of the coke-making process. The coal is crushed and ground into a powder and then charged into an oven where it is heated to approximately 1800°F in the absence of oxygen. As the oven is heated, the coal begins to melt so most of the volatile matter such as oil, tar, hydrogen, nitrogen and sulfur are removed. The cooked coal, called coke, is removed from the oven after 18 to 24 hours of reaction time. The coke is cooled and screened into pieces ranging from one inch to four inches. The coke is a porous, hard black rock of concentrated carbon (contains 90 to 93 percent carbon), which has some ash and sulfur but compared to raw coal is very strong. The strong pieces of coke with a high energy value provide permeability, heat and gases which are required to reduce and melt the iron ore, pellets and sinter. Today, natural gas is increasingly being added in place of coke to the same degree in the blast furnace to reduce carbon emissions
-
 1:06
1:06
Rumblestuff
6 days agoHow sewage water gets cleaned
270 -
 LIVE
LIVE
The Dan Bongino Show
2 hours agoBitter CNN Goes After Me (Ep. 2375) - 11/21/2024
149,513 watching -
 LIVE
LIVE
The Rubin Report
14 minutes agoCrowd Shocked by Ben Affleck’s Unexpected Take on This Massive Change
1,902 watching -
 LIVE
LIVE
Steven Crowder
2 hours ago🔴 BREAKING: Russia Launches ICBM for First Time in History - What Happens Next?
38,398 watching -
 1:18:10
1:18:10
Graham Allen
3 hours agoPutin Vows Peace With Trump But WAR Under Biden!! + 400,000 Kids Are MISSING?!
53.9K70 -
 2:11:07
2:11:07
Matt Kohrs
11 hours agoMSTR Squeezes Higher, Bitcoin To $100k & Nvidia Post Earnings || The MK Show
19K1 -
 42:07
42:07
BonginoReport
4 hours agoNikki Haley's Hatred of Tulsi Gabbard Just Made Me a Bigger Fan (Ep.90) - 11/21/24
59K162 -
 28:41
28:41
Professor Nez
10 hours ago🚨BLOOD on their HANDS! The Man Biden & Kamala Flew In Finally Faces JUSTICE for Laken Riley
29.6K18 -
 1:06:27
1:06:27
2 MIKES LIVE
2 hours agoThe Mike Schwartz Show 11-21-2024
7.96K -
 15:07
15:07
PMG
11 hours ago $0.13 earned"President Trump's Cabinet is Amazing!"
13.2K