Premium Only Content
Overview of Chemical Bonds
For Employees of hospitals, schools, universities and libraries: download up to 8 FREE medical animations from Nucleus by signing up for a free trial at: http://nmal.nucleusmedicalmedia.com/biology_youtube
#ChemicalBonds #IonicBonds #CovalentBonds
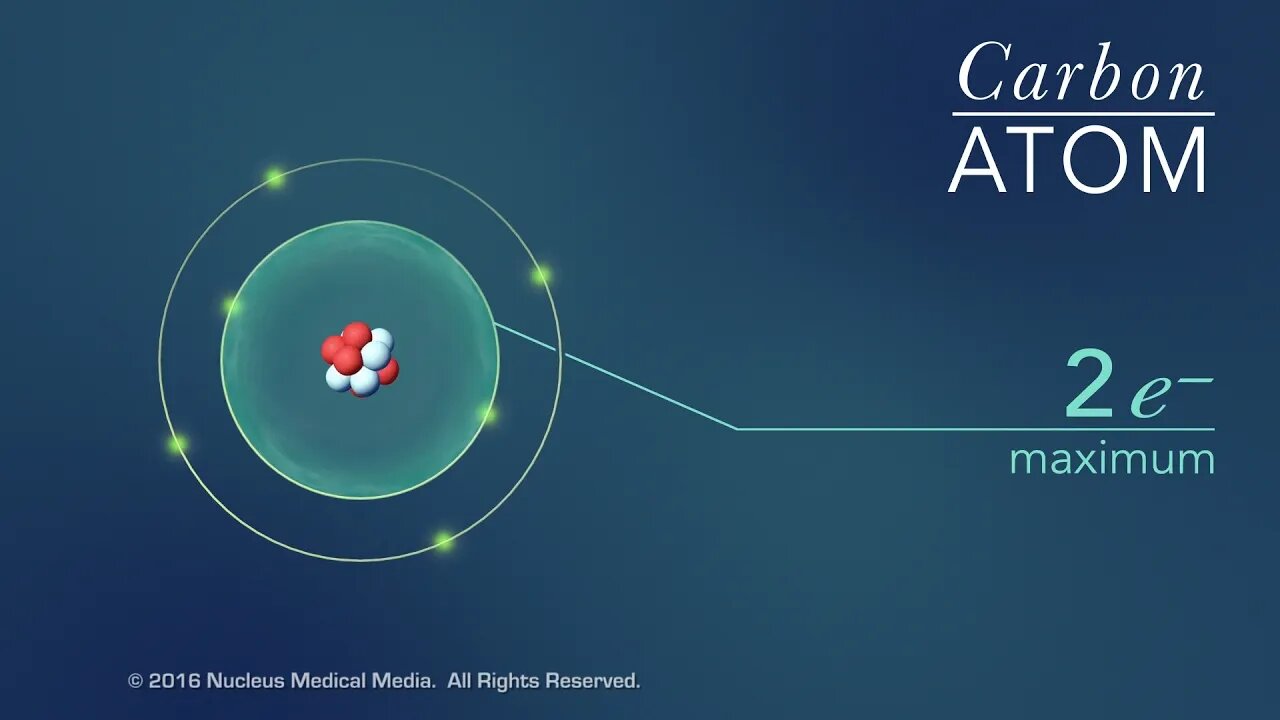
SCIENCE ANIMATION TRANSCRIPT: This video is an overview of chemical bonds. How do the atoms of elements form chemical bonds? Recall that electrons and an atom surround the nucleus. They feel energy levels or shells in specific numbers. Electrons in an atom's inner shells are commonly referred to as core electrons. Core electrons don't participate in chemical bonds. In contrast, electrons in the outermost shell of an atom are called valence electrons. Valence electrons do participate in forming chemical bonds. For example, a carbon atom with six protons and six neutrons in the nucleus has six electrons. Notice that carbon has both core and valence electrons. The innermost shell of any atom can hold a maximum of two electrons and the next shell can hold up to eight electrons. So, two of the electrons in carbon fill the first shell, and the remaining four electrons are in the next shell. The two electrons in the first shell are carbon's core electrons. The four electrons in the outer shell are carbon's valence electrons. Atoms with fewer valence electrons than its outer shell can hold aren't as stable as atoms with full outer shells. However, these atoms can become more stable if their outer shell is filled. This can happen either by loosing electrons to another atom or attracting electrons from another atom. This interaction of valence electrons between atoms results in the formation of chemical bonds. Elements that have completely filled outer shells such as helium are mostly non-reactive which means, they don't usually form chemical bonds. Why is that? Well, it's because their outer shells can't accept anymore electrons and losing all of their valence electrons requires too much energy. There are two main types of chemical bonds, they are ionic bonds, when electrons are transferred from one atom to another, and covalent bonds, when atoms share electrons. We'll discuss this in more detail separately. [music]
NSV16020
-
 LIVE
LIVE
Boxin
2 hours agoWe Start Tomb Raider I (Remastered)
64 watching -
 1:17:27
1:17:27
Man in America
15 hours agoEvidence of Highly Advanced Ancient Civilizations Is Being Hidden — But WHY!? w/ Jay Anderson
124K36 -
 8:00:05
8:00:05
SpartakusLIVE
14 hours agoDuos w/ GloryJean || Friday Night HYPE w/ The MACHINE
57.4K1 -
 3:47:23
3:47:23
Nerdrotic
16 hours ago $35.98 earnedFantastic 4 HER! Daredevil BORE Again SUCKS! Disney Star Wars is DESPERATE | FNT Vegas 350
161K30 -
 5:21:14
5:21:14
MyronGainesX
1 day ago $27.74 earnedFormer Fed Explains FSU Shooting, Charlie Kirk vs Groyper Debate!
123K43 -
 1:03:22
1:03:22
IsaacButterfield
14 hours ago $5.01 earnedKaty Perry in Space?! Trans Women Law Controversy & Lizzo's Weight Loss Shocks Fans!
59.2K18 -
 6:15:39
6:15:39
Sm0k3m
11 hours agogaming night
58.3K1 -
 3:48:35
3:48:35
I_Came_With_Fire_Podcast
19 hours agoHOUTHIS & CHINA | MURDERER MERCH STORE | TRUMP SICK OF WAITING
59.3K13 -
 1:09:15
1:09:15
Keepslidin
9 hours ago $1.24 earnedIRL GAMBLING & GIVING PEOPLE MONEY WHEN THEY WIN
33.5K5 -
 6:10:30
6:10:30
Eternal_Spartan
20 hours agoLive at 9pm Central | Halo 3 & Halo Firefight! Come Hang out with a USMC Vet and join the best chat!
33.2K1