Premium Only Content
Van der Waals Forces
For Employees of hospitals, schools, universities and libraries: download up to 8 FREE medical animations from Nucleus by signing up for a free trial at: http://nmal.nucleusmedicalmedia.com/biology_youtube
#VanDerWaals #molecules #MolecularAttraction
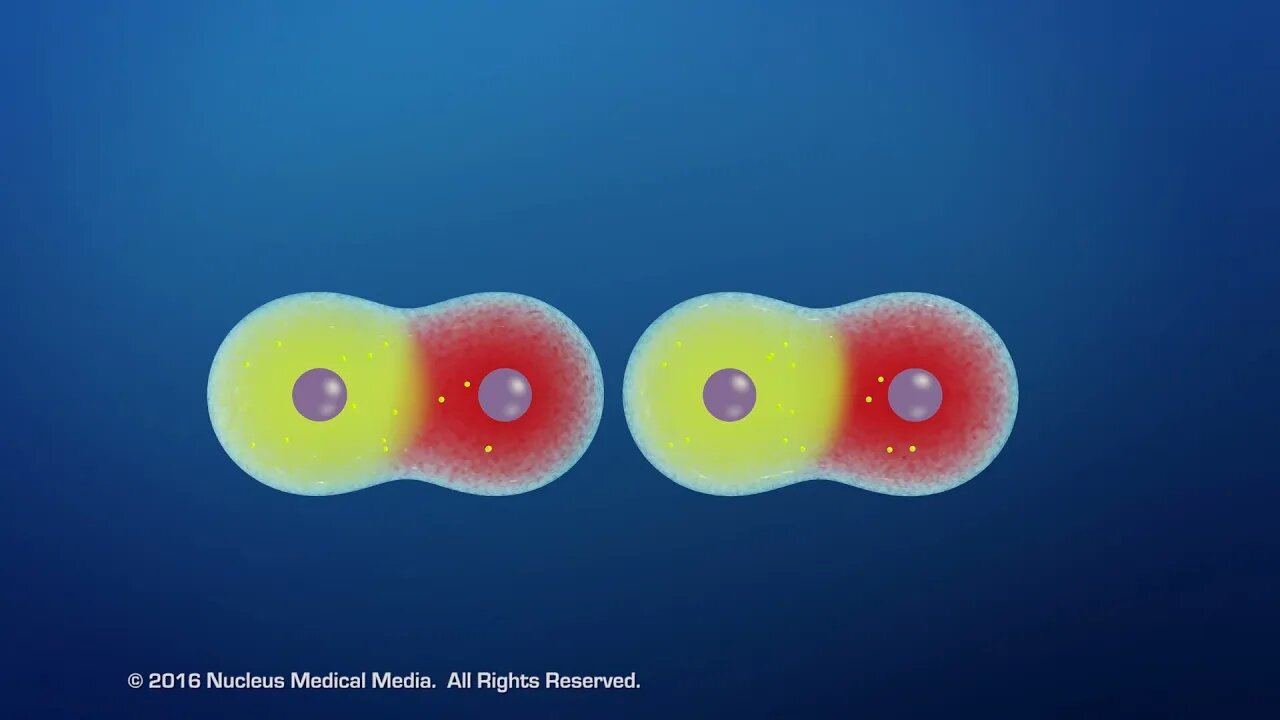
SCIENCE ANIMATION TRANSCRIPT: In this video, we'll discuss Van der Waals forces. Van der Waals forces are forces of attraction between molecules that are very close together. These forces between molecules are much weaker than the chemical bonds between the atoms holding a molecule together. Let's see how Van der Waals forces work. Molecules are electrically neutral because they have equal numbers of positively charged protons in the nucleus and negatively charged electrons outside the nucleus. In addition, some molecules are also polar. What does this mean? Well, polar molecules have permanent poles of electrical charge like a magnet because the electrons are unevenly distributed around the molecule. How does this happen? Let's look at an example of a polar molecule, water. A water molecule, or H2O, consists of two hydrogen atoms and one oxygen atom. When a water molecule forms, both hydrogen atoms bond with the oxygen atom by sharing their electrons with the oxygen atom. This completes both oxygen's outer electron shell, which can hold all eight electrons, and hydrogen's outer shell, which can hold two. However, the electrons aren't shared equally between the atoms because the oxygen atom attracts the electrons more strongly than hydrogen. As a result, a partial negative charge develops around oxygen because there are more negatively charged electrons around the oxygen side of the molecule. In comparison, fewer electrons around the hydrogen atoms create a partial positive charge on the hydrogen side of the molecule. This unequal sharing of electrons creates opposing poles of electrical charge on either side of the two bonds that hold the atoms together. Because of the opposite poles, these bonds are called polar covalent bonds. And since a water molecule is angled or bent with both of the hydrogen atoms on one side and the oxygen atoms on the other side, the molecule as a whole also has opposite poles and therefore is referred to as a polar molecule. Now, when polar molecules are near each other, a Van der Waals force of attraction between the molecules occurs because of their oppositely charged poles. In this example, the attraction of a polar molecule's negative pole to the positive pole around hydrogen atoms in water is a particularly strong type of Van der Waals force called a hydrogen bond. Hydrogen bonds only occur in polar molecules between hydrogen in one molecule and oxygen, nitrogen, and fluorine in the other. If a molecule doesn't have permanent poles of opposite electrical charge, it's called a non-polar molecule. However, non-polar molecules can become polar for very brief moments since the locations of electrons around atoms are constantly changing. This means the molecule can have a temporary negative pole on the side where there are momentarily more electrons, and a temporary positive pole on the opposite side where there are fewer electrons. The momentary concentration of electrons in this molecule's negative pole can repel the electrons in a nearby molecule toward its opposite end, making the neighboring molecule polar as well. The oppositely charged poles of adjacent molecules attract each other, forming weak connections between them called Van der Waals forces. Van der Waals forces explains two important properties: cohesion, the attraction between like molecules within a substance, and adhesion, the attraction between unlike molecules in different substances. An example of cohesion is when opposite poles of water molecules are attracted to each other but not to the surrounding air. This creates an inward force allowing water to bead up and form water droplets. Adhesion, the force of attraction between unlike molecules, explains how geckos are able to climb on slick, flat surfaces. Although each molecular connection is very weak, geckos can form millions of them between the molecules within the microscopic hairs on each foot and the molecules in the climbing surface. These connections add up to more than enough adhesion force to support the gecko's weight. In summary, Van der Waals forces are forces of attraction between molecules. They are not the same as chemical bonds between atoms within a molecule. They can occur in permanently polar molecules, such as water, and in non-polar molecules when they become briefly polar due to the changing positions of electrons. A hydrogen bond is a strong Van der Waals force between a polar molecule containing hydrogen atoms and the negative pole of another polar molecule. Van der Waals forces account for cohesion, the attraction between like molecules within a substance, and adhesion, the attraction between unlike molecules in different substances. [music]
NSV16025
-
 LIVE
LIVE
Scottish Viking Gaming
1 hour ago💚Rumble :|: Saturday Special :|: I LOVE THIS RUMBLE SHIT!!
104 watching -
 0:59
0:59
PewView
21 hours ago $4.39 earnedFound grandpas stash! 😂
12.6K4 -
 LIVE
LIVE
Shield_PR_Gaming
3 hours agoIt's Level Up Time! Bloodstrike and chatting
113 watching -
 21:00
21:00
Clownfish TV
18 hours agoNPR and PBS are SCREWED! They're Getting DEFUNDED?!
9.64K29 -
 34:44
34:44
LFA TV
4 days agoMIRACLES DO HAPPEN!
42K -
 LIVE
LIVE
Anvilight
3 hours agoLEGO Star Wars | Skywalker Saga Saturdays! | Creator Program Day #29
71 watching -
 LIVE
LIVE
Biscotti-B23
2 hours ago $0.23 earned🔴 LIVE GLOBKU'S $300 TOURNAMENT 🏆 COMMENTATING LATER ⚔ BLEACH REBIRTH OF SOULS
44 watching -
 LIVE
LIVE
TonYGaMinG
2 hours ago🟢 KHAZAN THE FIRST BERSERKER PART 2 #RumbleGaming
75 watching -
 19:08
19:08
Neil McCoy-Ward
22 hours agoHistory Is Repeating (Get Your House In Order NOW!)
11.4K4 -
 38:02
38:02
The Rich Dad Channel
19 hours agoSuccessfully Scale & Exit Your Business - Tom Wheelwright, Colin Campbell
10.1K