Premium Only Content
DR CARRIE MADEJ - TRANSGENE HYDRA EXPLAINED
DR CARRIE MADEJ - TRANSGENE HYDRA EXPLAINED
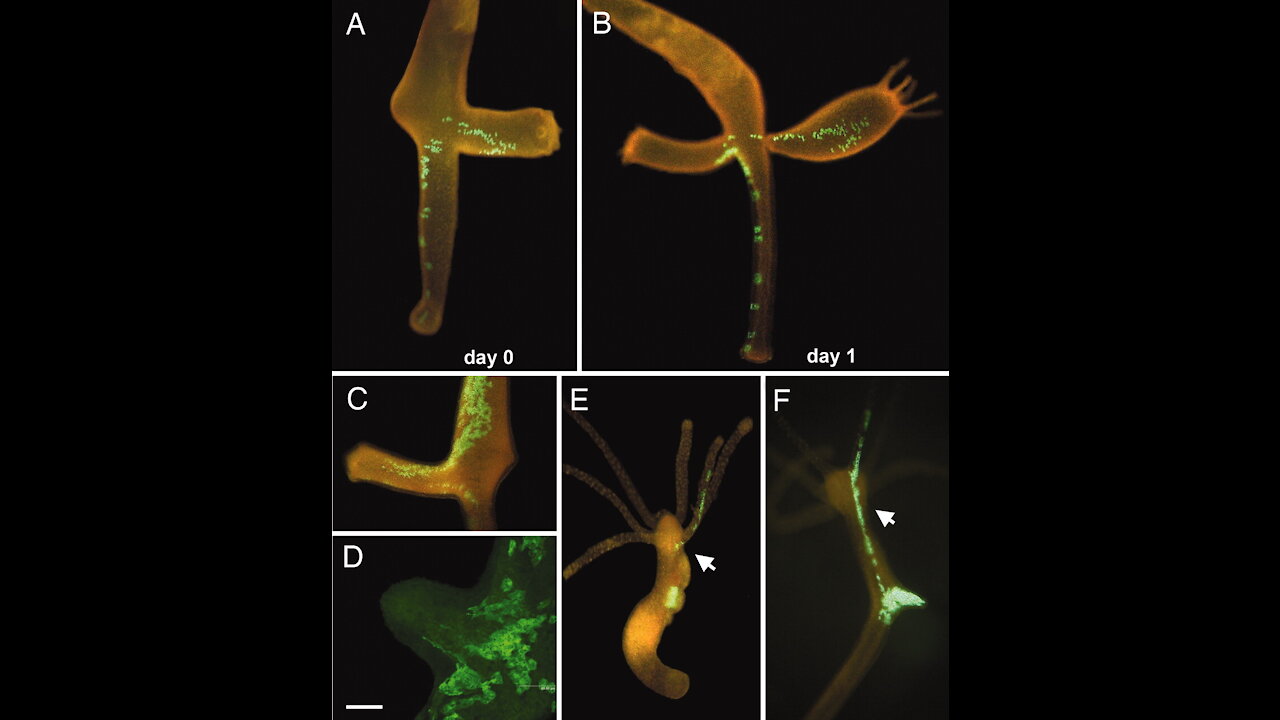
Transgenic Hydra Facility
Transgenic Hydra allow in vivo tracking of individual stem cells during morphogenesis
http://www.pnas.org/content/103/16/6208
Boundary maintenance in the ancestral metazoan Hydra depends on histone acetylation
Evolutionary crossroads in developmental biology: Cnidaria
Doctor: Hydras and Parasites in Vaxx.
Dr. Ariyana Love joined "The Stew Peters Show",
and made the case that the COV "vaXXines"
contain hydras and parasites,
and that they're being used to "transfect" humans into a "new species". During the interview,
Dr. Love begged inoculated people to NOT have children.
Therapeutic Cancer Vaccination with Ex Vivo RNA-Transfected Dendritic Cells—An Update
WNT/β-catenin signaling mediates human neural crest induction via a pre-neural border intermediate
https://pubmed.ncbi.nlm.nih.gov/26839343/
Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research
https://pubmed.ncbi.nlm.nih.gov/12648161/
Mass Transfer Phenomena in Electroporation
https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/electroporation
Nanoparticles versus Dendritic Cells as Vehicles to Deliver mRNA Encoding Multiple Epitopes for Immunotherapy
Introduction to Transfection | Thermo Fisher Scientific - US
https://www.sciencedirect.com/topics/neuroscience/transfection
mRNA Synthesis and Transfection
https://www.protocols.io/view/mrna-synthesis-and-transfection-9u5h6y6.html
Protocols for Synthetic mRNAs Drive Highly Efficient iPS Cell Differentiation to Dopaminergic Neurons
https://www.protocols.io/view/protocols-for-synthetic-mrnas-drive-highly-efficie-9e5h3g6
Nanoparticulate RNA delivery systems in cancer https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7941463/pdf/CNR2-3-e1271.pdf
Hydra Mesoglea Proteome Identifies Thrombospondin as a Conserved Component Active in Head Organizer Restriction
https://www.nature.com/articles/s41598-018-30035-2
High Efficiency mRNA Delivery
https://european-biotechnology.com/needful-things/products/product/high-efficiency-mrna-delivery.html
Hydra Mesoglea ProteomeIdentifes Thrombospondin as aConserved Component Active inHead Organizer Restriction
https://www.nature.com/articles/s41598-018-30035-2.pdf
Enlisting the mRNA VaXXine Platform to Combat Parasitic Infections
Biodistribution of in vivo-jetRNA-mRNA complexes via different administration routes
https://www.westburg.eu/products/cell-biology/transfection/in-vivo-transfection/in-vivo-mrna-delivery
Characterizing exogenous mRNA delivery, trafficking, cytoplasmic release and RNA-protein correlations at the level of single cells
Experimental attempt to produce mRNA transfected dendritic cells derived from enriched CD34+ blood progenitor cells
HYDRA VULGARIS
Molecular characterization of phospholipid hydroperoxide glutathione peroxidases from Hydra vulgaris
https://pubmed.ncbi.nlm.nih.gov/16919897/
An ancient chordin-like gene in organizer formation of Hydra
https://www.pnas.org/content/104/9/3249
Microinjection to deliver protein, mRNA, and DNA into zygotes of the cnidarian endosymbiosis model Aiptasia sp.
https://www.nature.com/articles/s41598-018-34773-1
Hydra meiosis reveals unexpected conservation of structural synaptonemal complex proteins across metazoans
https://www.pnas.org/content/109/41/16588
Hydra myc2, a unique pre-bilaterian member of the myc gene family, is activated in cell proliferation and gametogenesis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4021362/
Cloning of noggin gene from hydra and analysis of its functional conservation using Xenopus laevis embryos
https://pubmed.ncbi.nlm.nih.gov/20565537/
Deep sequencing reveals unique small RNA repertoire that is regulated during head regeneration in Hydra magnipapillata
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3592418/
NANOPARTICULATE DELIVERY SYSTEMSThe ability of NP to therapeutic agents, like siRNA or shRNA for the treatment of genetic disease, such as lung cancer, breast cancer, and Alzheimer's disease makes them a potent tool for gene therapy. 22 , 23 , 24 Their ability to mimic biological membranes and evade the immune system makes them ideal vehicles for drug delivery. The fabrication of a nanoparticulate delivery system, which can be utilized for more specific delivery of drugs has always been a priority for improved disease treatment.
Nanodimensional delivery systems came into existence in the late 1960s, 25 , 26 when researchers exploited the self‐assembling properties of block copolymers 26 and manufactured drug carrying, hollow vehicles. Self‐assembly is a thermodynamically driven and reversible phenomenon, where molecules form a well‐defined structure that is held together by weak, noncovalent forces. Self‐assembling properties of various block copolymers (or surfactants) have been exploited and used successfully to manufacture a biocompatible and biodegradable drug delivering vehicles. 27 , 28 , 29 , 30 Moreover, various shapes of NP can be attained by adjusting the packaging criteria of the self‐assembling molecules (or surfactants), (Figure (Figure1).1). In addition to manufacturing NP, it is also possible to design their shape according to the specific requirements. The purpose of the nanodimensional drug vehicles is to unload the encapsulated drug to the target site, which will further help in the treatment of disease. It is evident that many researchers have manufactured different drug delivery systems and successfully delivered the drug at the point of target, for instance, polyglycerol‐based dendrimers were produced and used by Paula Ofek et al against human glioblastoma and murine mammary adenocarcinoma cells. 31 , 32 , 33 , 34 , 35 The main challenge in drug delivery is to overcome the physiochemical and biological barriers such as the cell membrane, cell environment, blood serum protein, endosomal pathway, and immune system to allow successful drug administration. 36 Moreover, the biodegradable and biocompatible properties are equally important for the development of an effective NP. To achieve a NP associated with all the aforementioned properties, researchers have used different formulations of molecules or surfactants to create NP via various innovative methods.
mRNA-NP Transfection Efficiency in Various Cell Types (A) In vitro transfection efficiency of mRNA-NPs in BMDCs and stromal cell lines (LECs, FRCs, and DAPg7) after 48 h of culture using mCherry-encoding mRNA. The transfection efficiency of BM-DCs was significantly lower than any of the stromal cell lines (p % 0.01). (B-E) In vivo transfection efficiency of mRNA-NPs in different cell types using reporter mRNA in PLNs 48 h after i.p. injection. Data shown in panels B and C are from a study using GFP mRNA and no tissue digestion, while those depicted in panels D and E are from a study using mCherry mRNA and collagenase digestion to retrieve all APC populations. Control mice were untreated mice whose untransfected tissues/cells were used to determine fluorescence background for each cell type. The data are shown as mean of three biological replicates ± SEM (B and D) or as representative plots (C and E). Significant differences in uptake and expression of mRNA indicated for several cell types are relative to LNECs (ECs) for the treated group (D).
original link by PerthObserver
-
 39:49
39:49
Watchman's Duty
14 days agoIncandescent are back and light rabbit holes
3.08K13 -
 1:12:21
1:12:21
TheRyanMcMillanShow
23 hours ago $0.04 earnedDebbie Lee: Mother of First Navy Seal Killed In Iraq, Marc Lee - RMS 019
10K1 -
 38:05
38:05
Uncommon Sense In Current Times
15 hours ago $0.05 earnedIs Israel Being Forced Into a Bad Deal? David Rubin Exposes the Truth | Uncommon Sense
9.49K7 -

FreshandFit
10 hours agoAfter Hours w/ Girls
118K89 -
 2:33:58
2:33:58
TimcastIRL
13 hours agoDan Bongino ACCEPTS Deputy FBI Director, SECRET NSA CHATS EXPOSED w/Joey Mannarino | Timcast IRL
185K101 -
 1:09:33
1:09:33
Glenn Greenwald
17 hours agoMichael Tracey Reports from CPAC: Exclusive Interviews with Liz Truss, Steve Bannon & More | SYSTEM UPDATE #412
135K95 -
 56:02
56:02
Sarah Westall
13 hours agoBiohacking & Peptides: Weight loss, Anti-Aging & Performance – Myth vs Reality w/ Dr. Diane Kazer
76.3K33 -
 11:22
11:22
Bearing
23 hours ago"Anxious & Confused" Federal Workers FREAK OUT Over DOGE Efficiency Email 💥
100K79 -
 1:31:20
1:31:20
Flyover Conservatives
1 day agoUS STOCK MARKET: Sinking Ship - Dr. Kirk Elliott; How I Fought Back Against Woke Schools & Stopped Gender Bathrooms - Stacy Washington | FOC Show
90.9K5 -
 1:08:09
1:08:09
Donald Trump Jr.
18 hours agoFBI Dream Team, Plus Taking Your Questions Live! | Triggered Ep.219
234K304