Premium Only Content

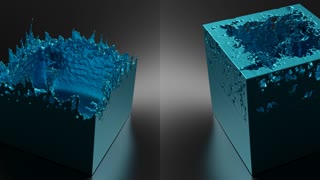
Liquid Metal that is Safe to Touch...and Play with.
Gallium is a chemical element with symbol Ga and atomic number 31. It is in group 13 of the periodic table, and thus has similarities to the other metals of the group, aluminum, indium, and thallium. Gallium does not occur as a free element in nature, but as gallium(III) compounds in trace amounts in zinc ores and in bauxite. Elemental gallium is a soft, silvery blue metal at standard temperature and pressure, a brittle solid at low temperatures, and a liquid at temperatures greater than 29.76 °C (85.57 °F) (slightly above room temperature). The melting point of gallium is used as a temperature reference point. The alloy galinstan (68.5% gallium, 21.5% indium, and 10% tin) has an even lower melting point of −19 °C (−2 °F), well below the freezing point of water.
Since its discovery in 1875, gallium has been used to make alloys with low melting points. It is also used in semiconductors as a dopant in semiconductor substrates.
Gallium is predominantly used in electronics. Gallium arsenide, the primary chemical compound of gallium in electronics, is used in microwave circuits, high-speed switching circuits, and infrared circuits. Semiconductive gallium nitride and indium gallium nitride produce blue and violet light-emitting diodes (LEDs) and diode lasers. Gallium is also used in the production of artificial gadolinium gallium garnet for jewelry.
Gallium has no known natural role in biology. Gallium(III) behaves in a similar manner to ferric salts in biological systems, and has been used in some medical applications, including pharmaceuticals and radiopharmaceuticals. Gallium is used in thermometers as a non-toxic and environmentally friendly alternative to mercury and can withstand higher temperatures than mercury.
In 1871, the existence of gallium was first predicted by Russian chemist Dmitri Mendeleev, who named it "eka-aluminium" from its position in his periodic table. He also predicted several properties of eka-aluminium that correspond closely to the real properties of gallium, such as its density, melting point, oxide character and bonding in chloride.
Mendeleev further predicted that eka-aluminium would be discovered by means of the spectroscope, and that metallic eka-aluminium would dissolve slowly in both acids and alkalis and would not react with air. He also predicted that M2O3 would dissolve in acids to give MX3 salts, that eka-aluminium salts would form basic salts, that eka-aluminium sulfate should form alums, and that anhydrous MCl3 should have a greater volatility than ZnCl2: all of these predictions turned out to be true.
Gallium was discovered using spectroscopy by French chemist Paul Emile Lecoq de Boisbaudran in 1875 from its characteristic spectrum (two violet lines) in a sample of sphalerite. Later that year, Lecoq obtained the free metal by electrolysis of the hydroxide in potassium hydroxide solution. He named the element "gallia", from Latin Gallia meaning Gaul, after his native land of France. It was later claimed that, in one of those multilingual puns so beloved by men of science in the 19th century, he had also named gallium after himself: "Le coq" is French for "the rooster" and the Latin word for "rooster" is "gallus". In an 1877 article, Lecoq denied this conjecture. Originally, de Boisbaudran determined the density of gallium as 4.7 g/cm3, the only property that failed to match Mendeleev's predictions; Mendeleev then wrote to him and suggested that he should remeasure the density, and de Boisbaudran then obtained the correct value of 5.9 g/cm3, that Mendeleev had predicted almost exactly.
From its discovery in 1875 until the era of semiconductors, the primary uses of gallium were high-temperature thermometrics and metal alloys with unusual properties of stability or ease of melting (some such being liquid at room temperature). The development of gallium arsenide as a direct band gap semiconductor in the 1960s ushered in the most important stage in the applications of gallium.
Elemental gallium is not found in nature, but it is easily obtained by smelting. Very pure gallium metal has a silvery color and its solid metal fractures conchoidally like glass. Gallium liquid expands by 3.1% when it solidifies; therefore, it should not be stored in glass or metal containers because the container may rupture when the gallium changes state. Gallium shares the higher-density liquid state with a short list of other materials that includes water, silicon, germanium, antimony, bismuth, and plutonium.
Music: Flying Dream by Dhruva Aliman
Amazon - https://amzn.to/2B9tGa7
https://music.apple.com/us/artist/dhruva-aliman/363563637
https://dhruvaaliman.bandcamp.com/album/road-of-fortunes
http://www.dhruvaaliman.com/
Spotify - https://open.spotify.com/artist/5XiFCr9iBKE6Cupltgnlet
#interesting
#science
#chemistry
-
 1:32
1:32
Kinetic CGI
3 years agoLiquid Metal
20 -
 7:38
7:38
Let's Play Everything
3 years agoLet's Play Everything: City Adventure Touch
35 -
 9:11
9:11
Space Ice
1 day agoFatman - Greatest Santa Claus Fighting Hitmen Movie Of Mel Gibson's Career - Best Movie Ever
121K48 -
 42:38
42:38
Brewzle
1 day agoI Spent Too Much Money Bourbon Hunting In Kentucky
82.2K13 -
 1:15:30
1:15:30
World Nomac
1 day agoMY FIRST DAY BACK in Manila Philippines 🇵🇭
63.9K9 -
 13:19
13:19
Dr David Jockers
1 day ago $11.93 earned5 Dangerous Food Ingredients That Drive Inflammation
82.5K17 -
 1:05:13
1:05:13
FamilyFriendlyGaming
1 day ago $15.97 earnedCat Quest III Episode 8
132K3 -
 10:39
10:39
Cooking with Gruel
2 days agoMastering a Succulent London Broil
84.6K5 -
 22:15
22:15
barstoolsports
1 day agoWhite Elephant Sends Barstool Office into Chaos | VIVA TV
59.7K1 -
 3:30:40
3:30:40
MrNellyGB
22 hours ago🔴LIVE - GRINDING MARVEL RIVALS RANKED! | #RumbleTakeover #RumblePremium
41.1K1