Premium Only Content
Corona Virus Vaccines and introduction
Leading COVID-19 vaccine candidates rely on new technologies that have fast-tracked development and testing. Vaccines from Pfizer and Moderna have completed early phase 3 clinical trials and are reportedly under review at the US FDA for emergency use authorization (EUA) although safety surveillance continues. This video explains the principles underlying the leading DNA, messenger RNA (mRNA), and viral vector vaccine candidates, and how they might induce immunity to SARS-CoV-2 infection.
0:00 Introduction
1:21 Traditional vaccines
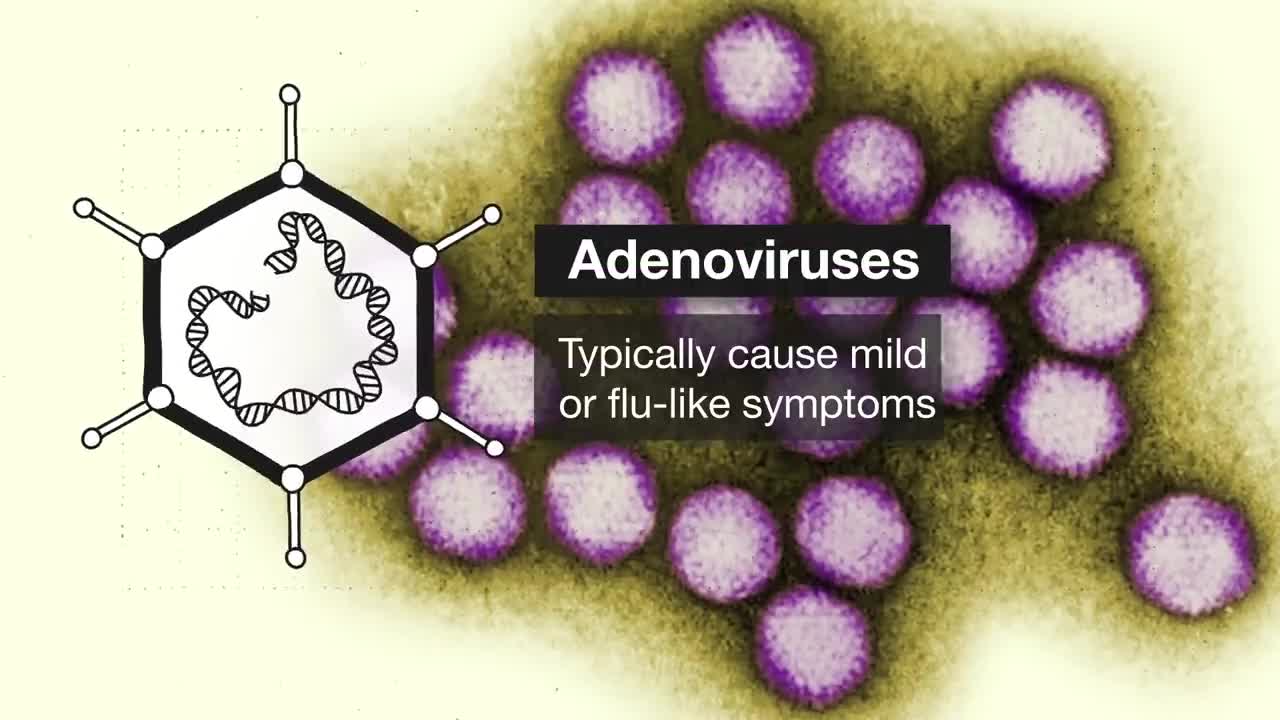
2:00 COVID-19 vaccine types in development
2:18 Making vaccines from a genetic sequence
2:45 Target antigen: the S protein
3:32 Genetic vaccines (DNA and mRNA)
4:18 Moderna/NIH and Pfizer/BioNTech vaccines
4:52 Viral vector vaccines
5:42 Adenovirus vectors (University of Oxford/AstraZeneca and Johnson & Johnson/Janssen Pharmaceuticals)
6:32 rVSV vector vaccine (Merck/IAVI)
7:15 Previous experience with next generation vaccines
7:50 Importance of Phase 3 Trials
-
 7:34:25
7:34:25
Dr Disrespect
22 hours ago🔴LIVE - DR DISRESPECT - WARZONE - IMPOSSIBLE TRIPLE THREAT CHALLENGE
236K35 -
 1:02:45
1:02:45
Tundra Tactical
12 hours ago $29.09 earned🛑 KASH PATEL NEW ATF DIRECTOR??? Breaking News!!!! 🛑
94.8K13 -
 4:31:10
4:31:10
I_Came_With_Fire_Podcast
22 hours agoMy EURO Divorce | HOGG with a side of PAC | Foreign FUNDS Fudged
59.4K9 -
 37:44
37:44
Glenn Greenwald
19 hours agoGlenn On Tearing Down the Military Industrial Complex, Exposing Pro-Israel Indoctrination, and More | SYSTEM UPDATE #411
138K199 -
 4:04:20
4:04:20
Nerdrotic
18 hours ago $55.57 earnedAmazon Takes 007! Hollywood is Lost, Disney Cancels WHO? | Friday Night Tights 342 /w ItsAGundam
190K55 -
 43:27
43:27
Tucker Carlson
17 hours agoRay Dalio: America’s Hidden Civil War, and the Race to Beat China in Tech, Economics, and Academia
213K205 -
 56:56
56:56
Candace Show Podcast
17 hours agoEXCLUSIVE: Taylor Swift Will Be Deposed. | Candace Ep 150
237K195 -
 1:03:52
1:03:52
IsaacButterfield
14 hours ago $10.07 earnedRepublican Vs 25 Transgender Activists | Jewish Outrage | Lizzo Loses All the Weight
81K23 -
 1:10:23
1:10:23
Edge of Wonder
18 hours agoChinese Biochips Hacking Minds? Quantum Control & Journey Song Mandela Effect
101K9 -
 2:15:46
2:15:46
Quite Frankly
21 hours ago"Ghosts, Robotics, and OBE's" ft. Dr. Albert Taylor 2/21/25
87.2K19