Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
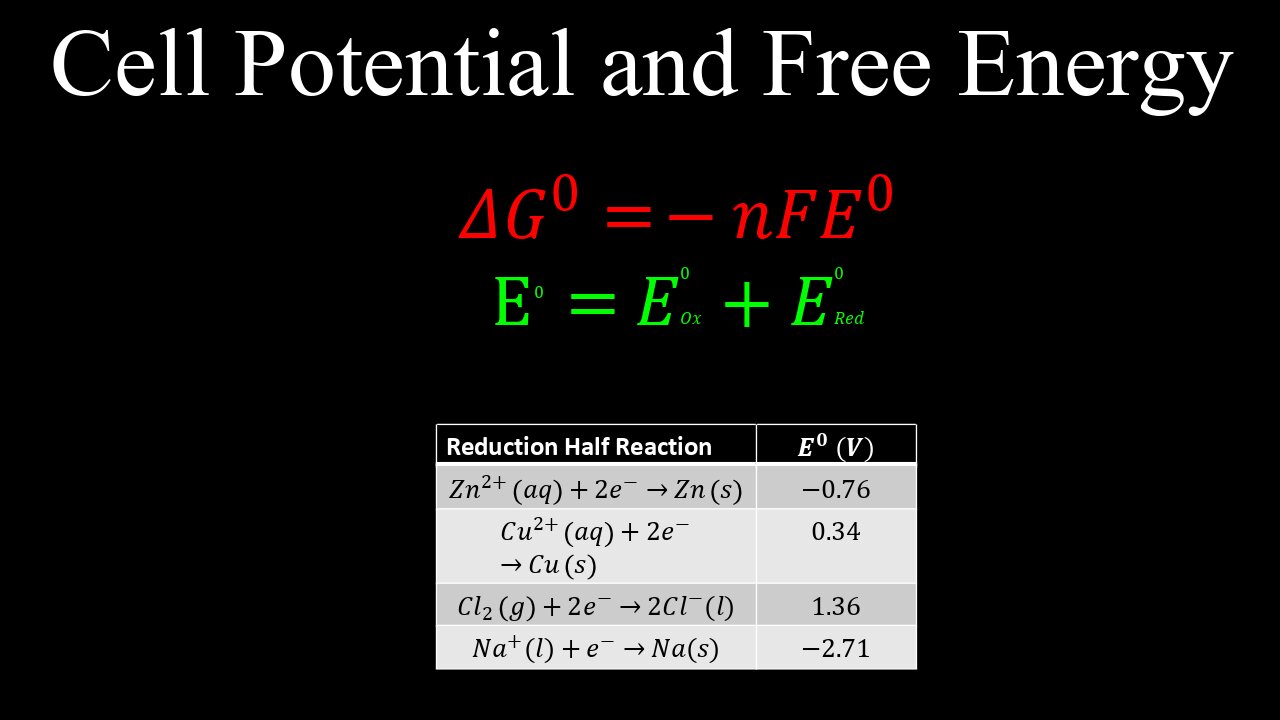
Cell Potential and Free Energy - Chemistry
1 day ago
137
Science
Education
cell potential and gibbs free energy
gibbs free energy
cell potential
chemistry
cell potential chemistry
relation between gibbs free energy and cell potential
cell potential electrical work and free energy
cell potential and gibbs free energy problem
gibbs free energy and cell potential
This video explains how the cell potential is related to the Gibbs Free Energy, including the equation (G=-nFE, where n is the number of moles of electrons and F is Faraday's constant, and E is the standard potential), and how it can be used to determine if a redox reaction is thermodynamically favourable.
0:00 Cell potential and free energy
3:32 Worked example
Loading 2 comments...
-
 1:02:11
1:02:11
The Dan Bongino Show
18 hours agoSunday Special with Vince Coglianese, Rep. Tim Burchett, Rep. Byron Donalds & Vivek Ramaswamy
173K264 -
 2:29:38
2:29:38
TheSaltyCracker
7 hours agoPiss Off War Pigs ReeEEeE Stream 03-09-25
154K301 -
 1:03:55
1:03:55
Sarah Westall
9 hours agoCanada Media Mind Control to increase Assisted Suicide, Confusion & Enslavement w/ Jasmin Laine
54.5K11 -
 2:41:11
2:41:11
Canal Paulo Figueiredo
2 days agoPedro Valente Debunks The Myths of Jiu-Jitsu History
44.4K7 -
 2:01:46
2:01:46
vivafrei
9 hours agoEp. 254: China to Pay $24 BILLION? Who Owns Embryos? Tulsi was RIGHT on Syria! Prorogation & MORE!
147K119 -
 3:40:55
3:40:55
MyronGainesX
19 hours ago $19.07 earnedFormer Fed Explains Gabby Petito's Murder
76.2K37 -
 2:18:05
2:18:05
Nerdrotic
9 hours ago $9.19 earnedInvestigations into the Unknown with Micah Hanks | Forbidden Frontier #093
74.2K16 -
 18:54
18:54
The Rubin Report
13 hours agoHow One Woman Outsmarted Pornhub & Exposed Its Dark Secrets | Laila Mickelwait
154K122 -
 LIVE
LIVE
Major League Fishing
5 days agoLIVE! - Bass Pro Tour: Stage 3 - Day 4
850 watching -
 1:05:28
1:05:28
Sports Wars
16 hours agoLebron GOES OFF Over Bronny Hate, Pereira LOSES Belt To Ankalaev At UFC 313, Xavier Worthy Arrested
105K19