Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
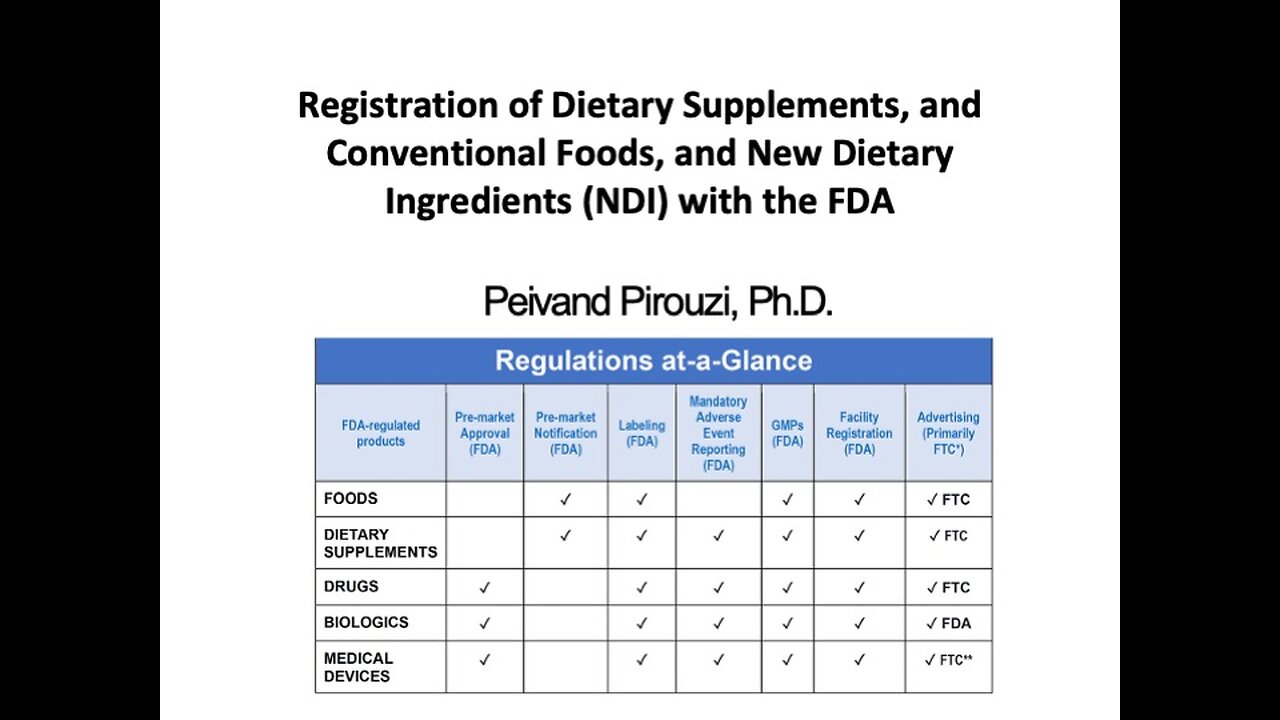
FDA Registration: Dietary Supplements, Conventional Foods, and NDIs by Peivand Pirouzi, Ph.D.
1 month ago
20
Explore the process of registering dietary supplements, conventional foods, and New Dietary Ingredients (NDI) with the FDA. This video provides a detailed guide on FDA requirements, including labeling, compliance, and safety evaluations for dietary supplements and NDIs. Learn the distinctions between dietary supplements and conventional foods, and understand the steps needed to navigate the regulatory landscape effectively. Whether you're a manufacturer, distributor, or stakeholder in the food and supplement industry, this video offers valuable insights to ensure compliance with FDA regulations. Stay informed and ensure your products meet regulatory standards!
Loading comments...
-
 7:34:25
7:34:25
Dr Disrespect
22 hours ago🔴LIVE - DR DISRESPECT - WARZONE - IMPOSSIBLE TRIPLE THREAT CHALLENGE
236K35 -
 1:02:45
1:02:45
Tundra Tactical
12 hours ago $30.48 earned🛑 KASH PATEL NEW ATF DIRECTOR??? Breaking News!!!! 🛑
94.8K14 -
 4:31:10
4:31:10
I_Came_With_Fire_Podcast
22 hours agoMy EURO Divorce | HOGG with a side of PAC | Foreign FUNDS Fudged
59.4K9 -
 37:44
37:44
Glenn Greenwald
19 hours agoGlenn On Tearing Down the Military Industrial Complex, Exposing Pro-Israel Indoctrination, and More | SYSTEM UPDATE #411
138K202 -
 4:04:20
4:04:20
Nerdrotic
18 hours ago $57.17 earnedAmazon Takes 007! Hollywood is Lost, Disney Cancels WHO? | Friday Night Tights 342 /w ItsAGundam
196K55 -
 43:27
43:27
Tucker Carlson
17 hours agoRay Dalio: America’s Hidden Civil War, and the Race to Beat China in Tech, Economics, and Academia
213K210 -
 56:56
56:56
Candace Show Podcast
18 hours agoEXCLUSIVE: Taylor Swift Will Be Deposed. | Candace Ep 150
248K200 -
 1:03:52
1:03:52
IsaacButterfield
14 hours ago $10.86 earnedRepublican Vs 25 Transgender Activists | Jewish Outrage | Lizzo Loses All the Weight
86.4K23 -
 1:10:23
1:10:23
Edge of Wonder
18 hours agoChinese Biochips Hacking Minds? Quantum Control & Journey Song Mandela Effect
101K9 -
 2:15:46
2:15:46
Quite Frankly
21 hours ago"Ghosts, Robotics, and OBE's" ft. Dr. Albert Taylor 2/21/25
90.1K19