Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
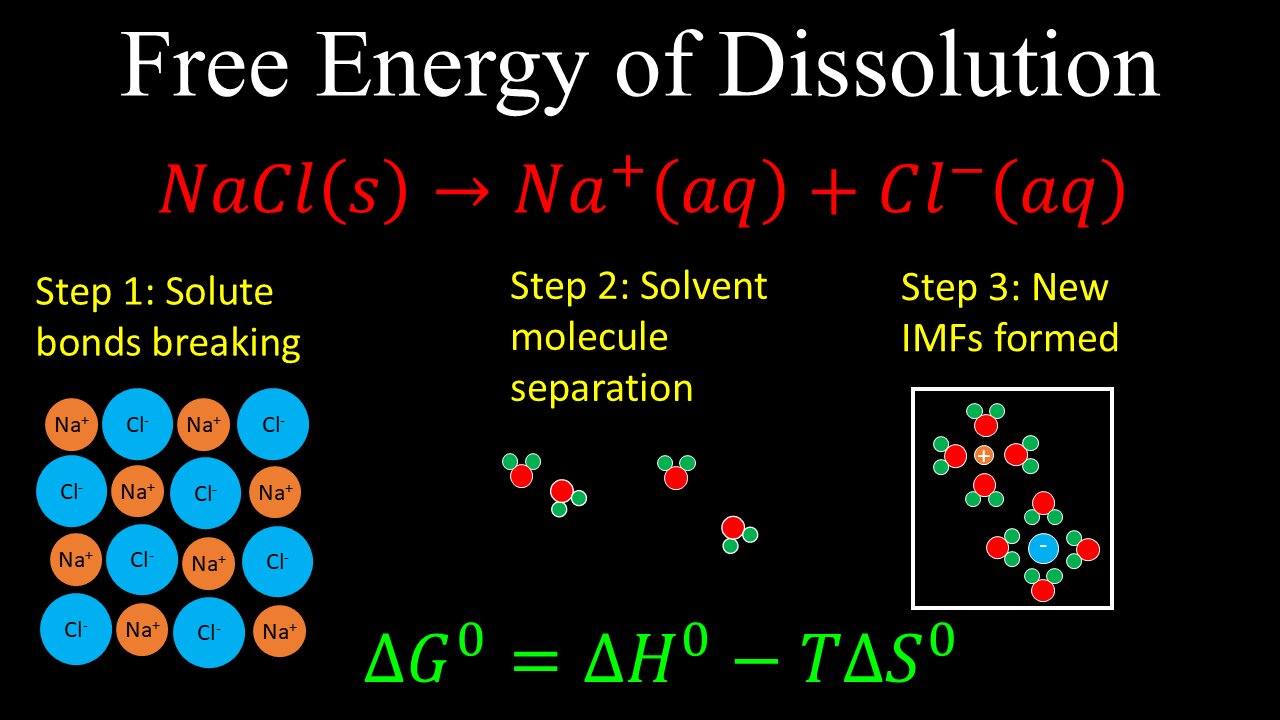
Free Energy of Dissolution, Enthalpy, Entropy, Example - AP Chemistry
1 day ago
26
Science
Education
gibbs free energy
chemistry
enthalpy
free energy of dissolution practice
entropy
chemistry (field of study)
ap chemistry
enthalpy entropy thermodynamically favored processes
general chemistry (field of study)
This video explains how the standard enthalpy and entropy (which are related by the Gibbs free energy) change during the steps of dissolution of an ionic compound (e.g. sodium chloride), which includes the breaking of solute bonds, separation of the solvent molecules and formation of new intermolecular forces.
Loading comments...
-
 LIVE
LIVE
Graham Allen
2 hours agoBiden is SABOTAGING Trump!! Says: “Our Economy Is Strong” + Elon Is Buying TikTok?!
5,889 watching -
 LIVE
LIVE
Matt Kohrs
11 hours agoPPI Inflation Report: Stocks Rip Higher (DJT, NVDA & TSLA) || The MK Show
1,266 watching -
 LIVE
LIVE
Wendy Bell Radio
4 hours agoAmerica Needs To Be Democrat-Proofed
11,563 watching -
 LIVE
LIVE
2 MIKES LIVE
11 hours agoTHE MIKE SCHWARTZ SHOW with DR. MICHAEL J SCHWARTZ 01-14-2025
99 watching -
 19:15
19:15
Clownfish TV
11 hours agoElon Musk Buying TikTok?! TikTok Users FLEE to RedNote!
5653 -
 26:35
26:35
BonginoReport
4 hours agoCarrie Underwood Shakes Off the Cancel Mob’s Wrath (Ep.118) - 01/14/2025
22.2K32 -
 LIVE
LIVE
Vigilant News Network
15 hours agoBone-Chilling “Conspiracy Theory” Emerges as California Burns | The Daily Dose
1,140 watching -
 1:27:16
1:27:16
Game On!
10 hours ago $2.98 earnedJerry Jones SHOCKS the world! Ready to make Deion Sanders head coach of the Dallas Cowboys!
21.7K4 -
 8:50
8:50
Space Ice
17 hours agoSteven Seagal's Sniper: Special Ops Is His Greatest Achievement - Worst Movie Ever
43.7K25 -
 14:57
14:57
MichaelBisping
16 hours agoBISPING: The Complete History UFC's Lightweight Division | "Will ISLAM BECOME GOAT after UFC 311?"
67.2K1