Premium Only Content
Regulatory Affairs - Health Canada Clinical Trial Application CTA in CTD format - Completing form 3011. P.Pirouzi, Ph.D.
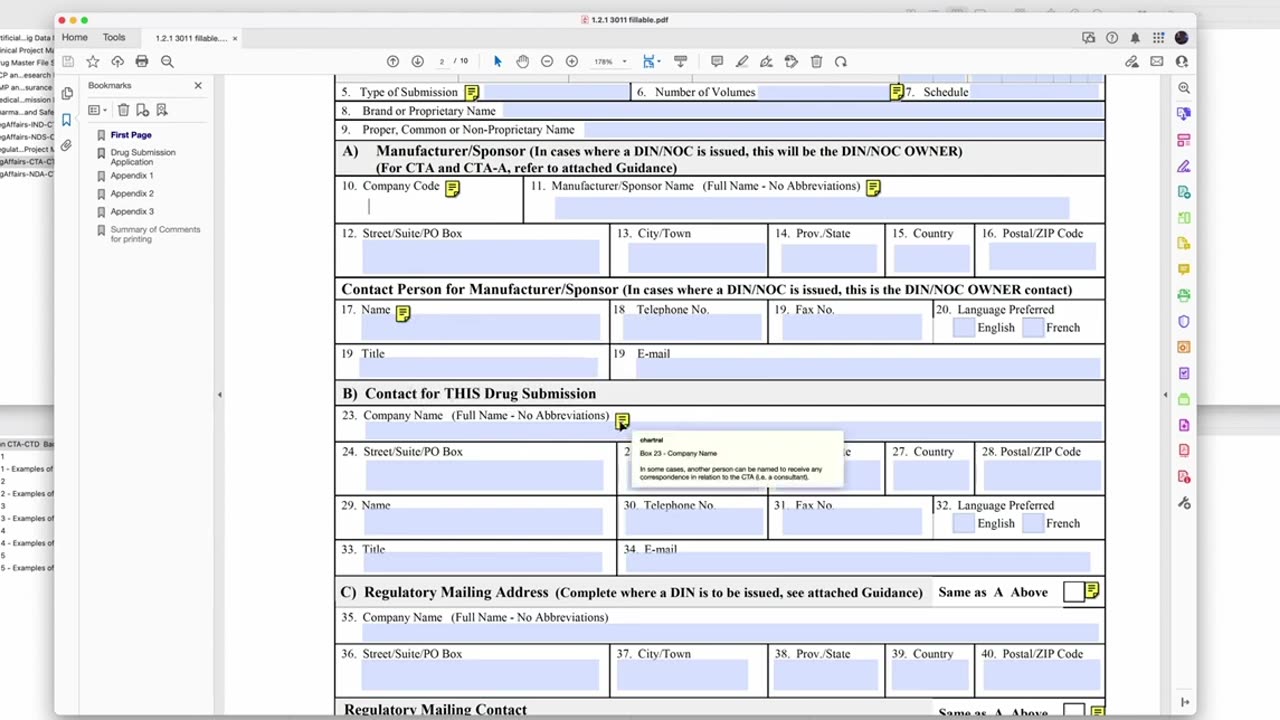
In this video, Professor Peivand Pirouzi provides a comprehensive guide to the Clinical Trial Application (CTA) process in Canada, specifically focusing on Form 3011 within the Common Technical Document (CTD) format. This form is a critical part of submitting a clinical trial application to Health Canada for approval, and understanding how to properly complete it is essential for researchers, sponsors, and regulatory affairs professionals.
Key topics covered include:
An overview of Health Canada's CTA process and the importance of submitting a well-prepared application.
A step-by-step demonstration of how to complete Form 3011 within the CTD format for clinical trial submission.
Key sections of the form and how to correctly fill in the required information for regulatory compliance.
Common pitfalls to avoid when completing the CTA and tips for ensuring a smooth approval process.
Best practices for clinical trial sponsors in preparing all necessary documentation for Health Canada's review.
This video is a valuable resource for anyone involved in clinical trial submissions in Canada, including researchers, clinical trial managers, and regulatory professionals looking to understand the process and complete Form 3011 effectively.
-
 4:15
4:15
Tactical Advisor
5 days agoWraithworks New Guns | Shot Show 2025
327 -
 18:16
18:16
Bearing
22 hours ago"If You Booed Taylor You Are a LOSER" - Taylor Swift Fans Are MAD 😡
3.34K44 -
 43:45
43:45
The Finance Hub
15 hours ago $0.08 earnedI CAN'T BELIEVE WHAT JUST HAPPENED TO CHUCK SCHUMER!
1252 -
 12:23
12:23
MTNTOUGH Fitness Lab
1 day agoMTNTOUGH Lab Visit: Remi Warren | Bad BBQ And The El Pres Drill
1.13K1 -
 49:58
49:58
Sarah Westall
11 hours agoTrump's $500 Billion Stargate Initiative, AI Singularity, Fear Porn & More w/ Patrick Hedger
8.82K -
 1:00:06
1:00:06
Trumpet Daily
20 hours ago $3.59 earnedAuditing the Swamp - Trumpet Daily | Feb. 12, 2025
4.55K11 -
 32:44
32:44
Jamie Kennedy
13 hours agoEp. 191 How To Deal with Users...
1.98K -
 56:37
56:37
PMG
1 day ago"The Last Chance to Save Our Republic!"
2.48K -
 3:36:49
3:36:49
Alex Zedra
10 hours agoLIVE! Scary Games Girls Night
50.2K7 -
 3:48:42
3:48:42
FreshandFit
9 hours agoGirl Has A Sugar Daddy And Another Claims 8 Months Of Celibacy?
167K91