Premium Only Content
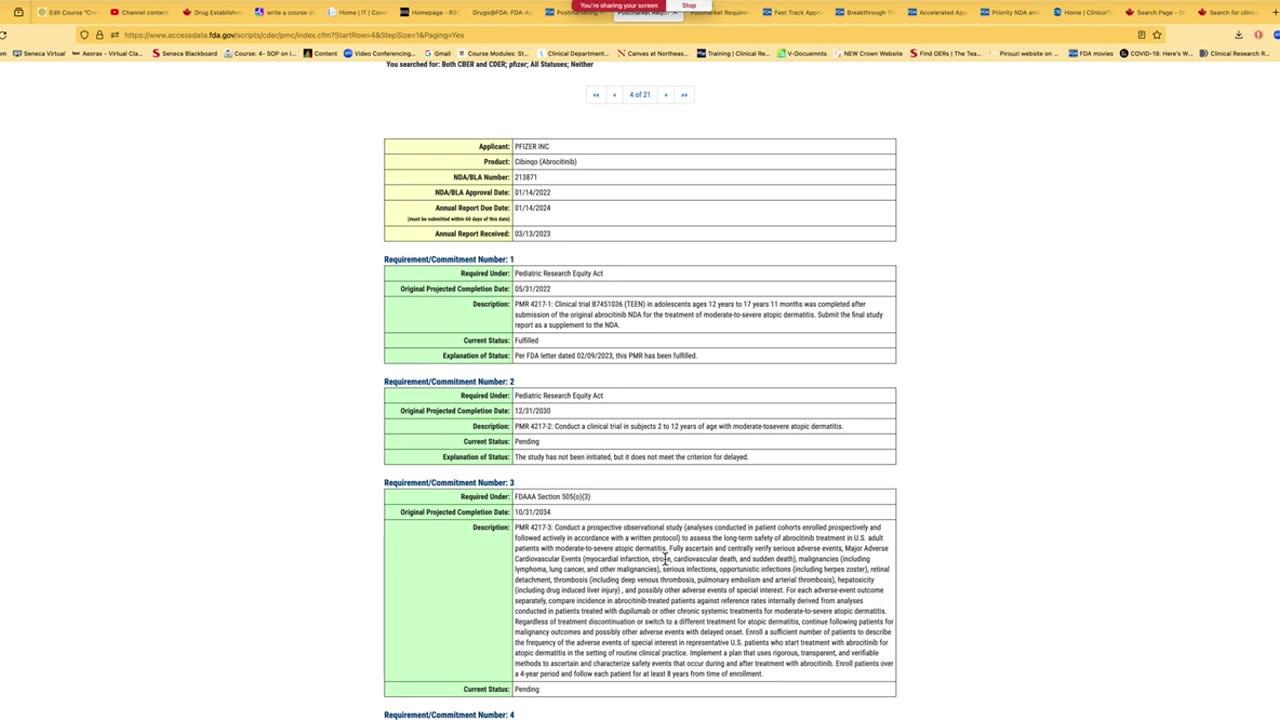
Regulatory Affairs - FDA databases: Drugs@FDA and Clinical trials.gov - Live demonstrations - Peivand Pirouzi, Ph.D.
In this video, Professor Peivand Pirouzi provides live demonstrations of two essential FDA databases: Drugs@FDA and ClinicalTrials.gov. These databases are crucial resources for researchers, clinicians, and healthcare professionals involved in drug development, regulatory affairs, and clinical trial management.
Professor Pirouzi walks through the functionalities of both databases, showcasing how they can be used to access detailed information about FDA-approved drugs, clinical trials, and regulatory processes.
Key topics covered include:
An introduction to the Drugs@FDA database and how to find information about FDA-approved drugs, their approval history, labeling, and more.
A detailed demonstration of ClinicalTrials.gov, a vital resource for finding clinical trial data, study results, and trial information.
Tips on how to effectively navigate these databases for research, regulatory purposes, and clinical trial analysis.
Insights into the role of these databases in supporting evidence-based practice and drug development.
Whether you're a researcher, clinical trial manager, regulatory affairs specialist, or healthcare professional, this video provides essential guidance on utilizing these critical FDA resources for your work.
-
 2:47:25
2:47:25
Right Side Broadcasting Network
13 hours agoLIVE REPLAY: President Donald J. Trump Holds First Press Briefing Since Inauguration - 1/21/25
242K211 -
 1:08:45
1:08:45
Man in America
12 hours agoTrump UNLEASHED! Dismantling the Deep State and Restoring America
44.8K46 -
 27:22
27:22
I_Came_With_Fire_Podcast
14 hours ago🔥SPECIAL RELEASE🔥 Inflation Reduction Act: American Seniors Get SLAMMED!!
47.7K10 -
 6:25:28
6:25:28
vivafrei
12 hours agoD.C. Gulag Jan. 6 Prisoners Release Watch!
221K111 -
 1:49:14
1:49:14
Redacted News
11 hours agoTrump is Back! Congress Uncovers New Biden Crimes One Day After He Leaves D.C. | Redacted
197K252 -
 2:09:53
2:09:53
Benny Johnson
11 hours ago🚨President Trump LIVE Right Now Making MASSIVE Announcement At White House News Conference
300K434 -
 2:04:10
2:04:10
Revenge of the Cis
12 hours agoEpisode 1433: Retribution
131K21 -
 1:42:50
1:42:50
The Criminal Connection Podcast
16 hours ago $1.26 earnedEddie Hearn talks JOSHUA vs FURY, Working With Frank Warren & The Truth About Turki Alalshikh!
65.9K2 -
 1:00:25
1:00:25
In The Litter Box w/ Jewels & Catturd
1 day agoGolden Age | In the Litter Box w/ Jewels & Catturd – Ep. 724 – 1/21/2025
154K83 -
 57:42
57:42
The Dan Bongino Show
19 hours agoHE'S BACK! (Ep. 2405) - 01/21/2025
1.45M2.66K