Premium Only Content
Regulatory Affairs - Clinical Trials Information System in Europe (EMA-Clinical Research). Peivand Pirouzi, Ph.D.
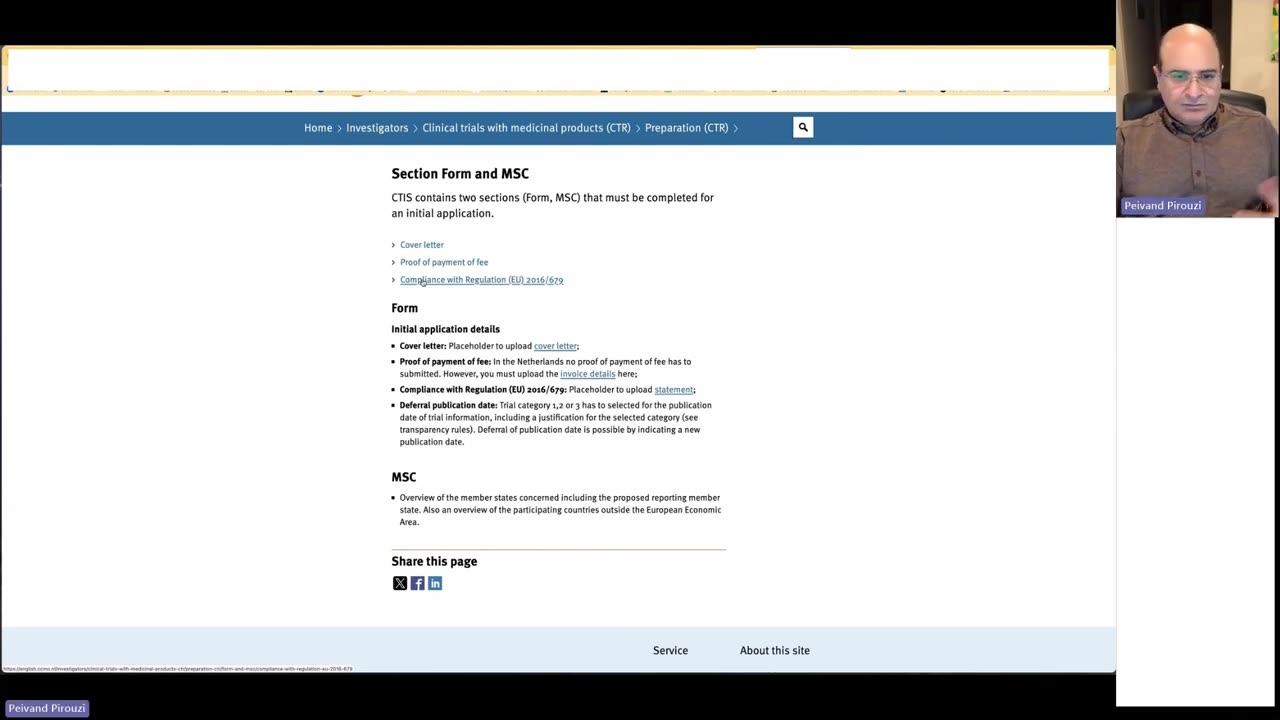
In this video, Professor Peivand Pirouzi provides an in-depth look at the Clinical Trials Information System (CTIS), a crucial initiative by the European Medicines Agency (EMA) that aims to streamline and enhance the management of clinical trials across Europe. With the implementation of this system, the regulatory environment for clinical research in Europe is evolving, providing new opportunities and challenges for researchers and sponsors alike.
Key topics covered include:
An overview of the Clinical Trials Information System (CTIS) and its role in clinical research
How the EMA is improving the submission, assessment, and supervision of clinical trials through CTIS
Key features and functionalities of the CTIS platform for clinical trial sponsors and researchers
Navigating regulatory requirements and compliance in clinical research within Europe
The impact of CTIS on transparency, patient safety, and data management
This video is a valuable resource for researchers, clinical trial managers, and regulatory affairs professionals working in Europe or with European clinical trials. Professor Pirouzi provides insights into how to leverage CTIS for effective and efficient clinical trial management.
-
 12:26
12:26
EvenOut
10 hours ago $8.45 earnedWe Got Cosmestic Surgery That Went Wrong! Twin Switch-Up!
33.7K6 -
 1:15:07
1:15:07
Ami's House
16 hours ago $5.85 earnedWhat an ACTUAL Military Expert Thinks of the War in Gaza – Nick Freitas | FULL EP
20.4K13 -
 2:54:02
2:54:02
TimcastIRL
8 hours agoLiberal Media CAUGHT In BOGUS LEAK, Trump DID NOT Fire Mike Waltz, HE PROMOTED HIM | Timcast IRL
208K124 -
 9:05:24
9:05:24
MyronGainesX
15 hours ago $24.43 earnedSam Seder Embarrasses Ethan Klein, The Truth On MLK's Murder, Panel Debate w/ Anton Daniels
74.7K17 -
 1:09:38
1:09:38
Man in America
11 hours agoEXPOSED: How Militaries Worldwide Are Engineering DEPOPULATION w/ Todd Callender
62.1K37 -
 DVR
DVR
SpartakusLIVE
10 hours agoNEW Update, NEW Weapons, NEW META?!? || Quads in VERDANSK
26.6K -
 5:14:28
5:14:28
Jokeuhl Gaming and Chat
7 hours agoEmpyrion - Galactic Survival Long Range Jump Aquired
33.3K1 -
 4:42:30
4:42:30
Right Side Broadcasting Network
1 day agoLIVE REPLAY: President Trump Gives Commencement Address at University of Alabama - 5/1/25
162K21 -
 16:58
16:58
T-SPLY
14 hours agoDems’ Bad News: El Salvador Rejects Abrego, Democrats Fume!
96.6K61 -
 18:56
18:56
Nick Shirley
9 hours ago $6.04 earnedAsking People About Trump’s First 100 Days… How are Americans Feeling?
37.9K42