Premium Only Content
Regulatory Affairs - Health Canada Form-0292-Medical Devices Establishment Licensing (MDEL) Application. P.Pirouzi, Ph.D.
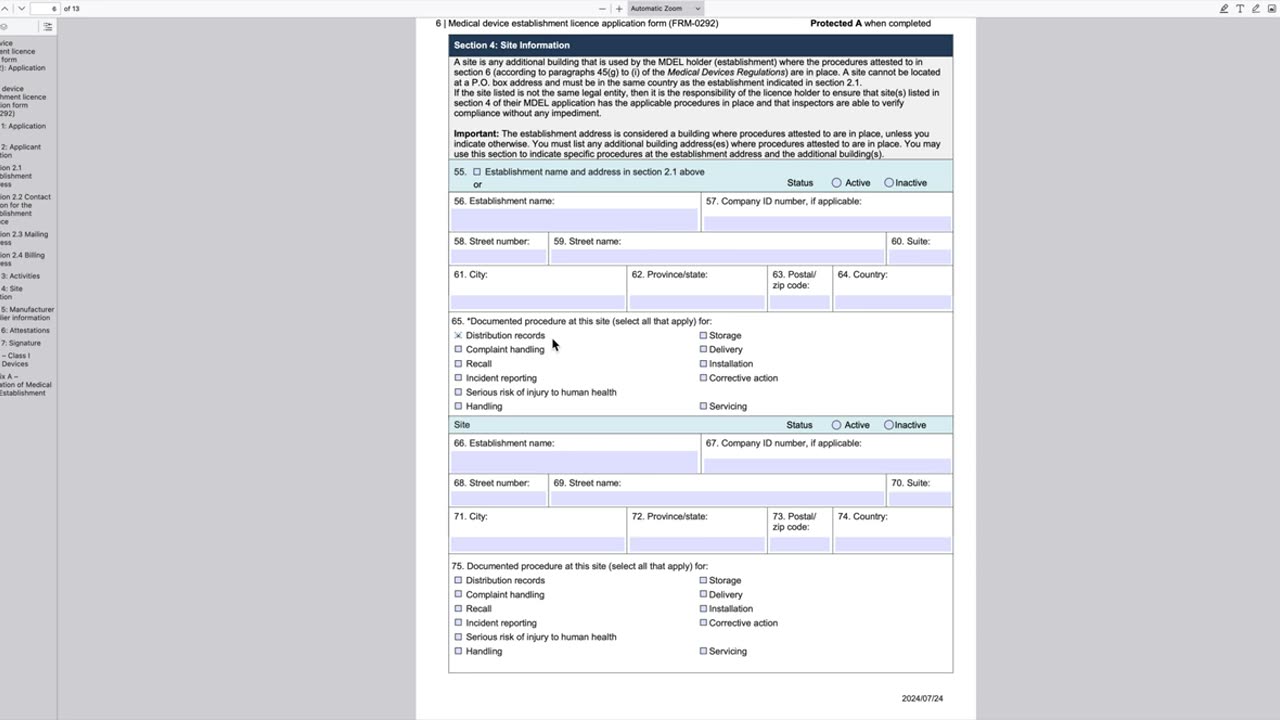
In this video, Professor Peivand Pirouzi walks you through Health Canada Form-0292, the Medical Devices Establishment Licensing (MDEL) application process. This is a critical step for companies involved in the sale or distribution of medical devices in Canada, ensuring compliance with Health Canada's regulatory requirements.
Professor Pirouzi covers the following key aspects:
An overview of the MDEL application process
Detailed explanation of Form-0292, including required information and documentation
The role of the MDEL in ensuring the safety and efficacy of medical devices sold in Canada
Step-by-step guidance on completing and submitting the MDEL application
Common challenges and tips for successful submission
Whether you're a regulatory affairs professional, a medical device manufacturer, or someone seeking to understand the licensing process in Canada, this video provides essential guidance to navigate the MDEL application and ensure your business remains compliant with Canadian regulations.
-
 LIVE
LIVE
Jeff Ahern
1 hour agoNever Woke Wednesday with Jeff Ahern (6am pacific)
209 watching -
 10:45
10:45
EvenOut
13 hours ago $1.54 earnedThe Non-Reflecting Mirror On Omegle Twin Prank!
17.3K3 -
 20:07
20:07
Scammer Payback
15 hours ago$4,000,000 Trap set on a Scammer
63.9K5 -
 5:45
5:45
Tactical Advisor
1 day agoWoox New Lever Action | Shot Show 2025
24.5K3 -
 16:25
16:25
Clownfish TV
13 hours agoDEI is Deader Than Disco! Hollywood Most Affected?!
20.9K5 -
 1:04:23
1:04:23
CarlCrusher
15 hours agoThe TRUTH about Roswell and the UFO Crash Recovery Material
16.6K4 -
 59:40
59:40
Trumpet Daily
21 hours ago $4.52 earnedAmerica’s Royal Family - Trumpet Daily | Jan. 21, 2025
17.5K21 -
 1:03:14
1:03:14
Bek Lover Podcast
14 hours agoInteresting Times with Bek Lover Podcast
40.2K6 -
 3:10:10
3:10:10
Price of Reason
16 hours agoTrump is BACK in Action! Elon Musk's Inauguration Gesture Draws MSM Fire! Disney Prepares For LOSSES
50.9K8 -
 2:08:22
2:08:22
Kim Iversen
16 hours agoIs This Even Legal? Trump’s Push to End Birthright Citizenship and The Insane Plan to Move Palestinians to Indonesia
147K317