Premium Only Content
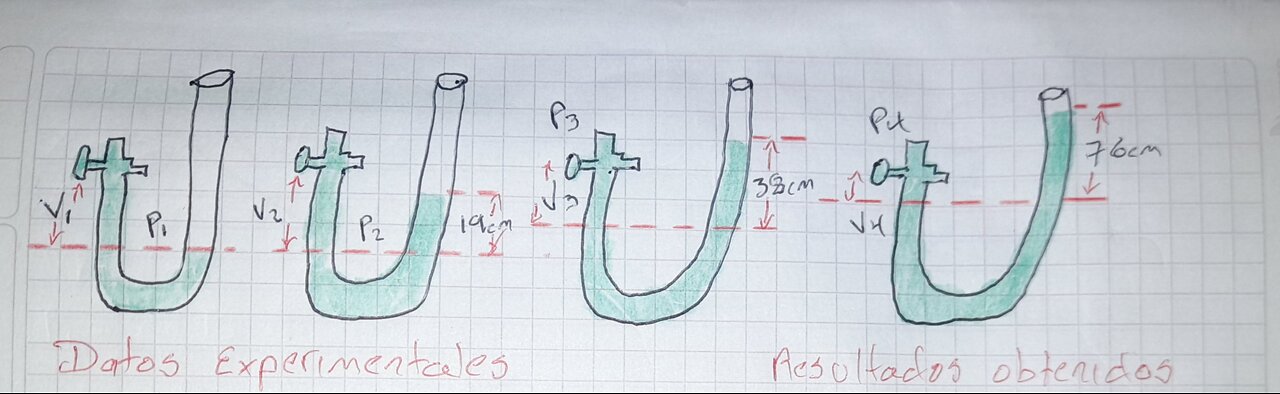
ISOTHERMAL LAW: EXPERIMENT WITH REAL RESULTS IN A CHEMICAL LABORATORY
Boyle's Law is a fundamental law in physics and chemistry that describes the relationship between the pressure and volume of a gas at constant temperature.
Statement of Boyle's Law
"The pressure of a gas is inversely proportional to the volume, as long as the temperature remains constant."
mathematical formula
P1V1 = P2V2
where:
1. P1 and P2 are the initial and final pressures
2. V1 and V2 are the initial and final volumes
Examples
1. If the volume of a gas is reduced by half, the pressure doubles.
2. If the pressure of a gas is doubled, the volume is reduced by half.
Applications
1. Artificial respiration
2. Air compressors
3. Vacuum pumps
4. Cooling systems
5. Scientific research
Limitations
1. The law only applies to ideal gases
2. Does not apply to liquids or solids
3. The temperature must be constant
Relationship with other laws
1. Charles's Law (relationship between volume and temperature)
2. Gay-Lussac's law (relationship between pressure and temperature)
3. Ideal gas law (combines the laws of Boyle, Charles and Gay-Lussac)
-
 1:47:46
1:47:46
SpartakusLIVE
10 hours agoThe Master RIZZLER has entered the building, the 95% REJOICE
27.4K2 -
 29:53
29:53
MYLUNCHBREAK CHANNEL PAGE
1 day agoOff Limits to the Public - Pt 1
86.7K110 -
 16:03
16:03
Tundra Tactical
12 hours ago $16.09 earnedNew Age Gun Fudds
123K20 -
 8:22
8:22
Russell Brand
16 hours agoThey want this to happen
205K393 -
 2:06:43
2:06:43
Jewels Jones Live ®
1 day ago2025 STARTS WITH A BANG! | A Political Rendezvous - Ep. 104
114K41 -
 4:20:41
4:20:41
Viss
16 hours ago🔴LIVE - PUBG Duo Dominance Viss w/ Spartakus
90K10 -
 10:15:14
10:15:14
MDGgamin
19 hours ago🔴LIVE-Escape From Tarkov - 1st Saturday of 2025!!!! - #RumbleTakeover
72K2 -
 3:54:19
3:54:19
SpartakusLIVE
15 hours agoPUBG Duos w/ Viss || Tactical Strategy & HARDCORE Gameplay
81.8K1 -
 5:54:54
5:54:54
FRENCHY4185
16 hours agoFRENCHY'S BIRTHDAY BASH !!! THE BIG 40 !!!
89.9K3 -
 1:23:33
1:23:33
Michael Franzese
1 day agoThings to look forward to in 2025
107K63