Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
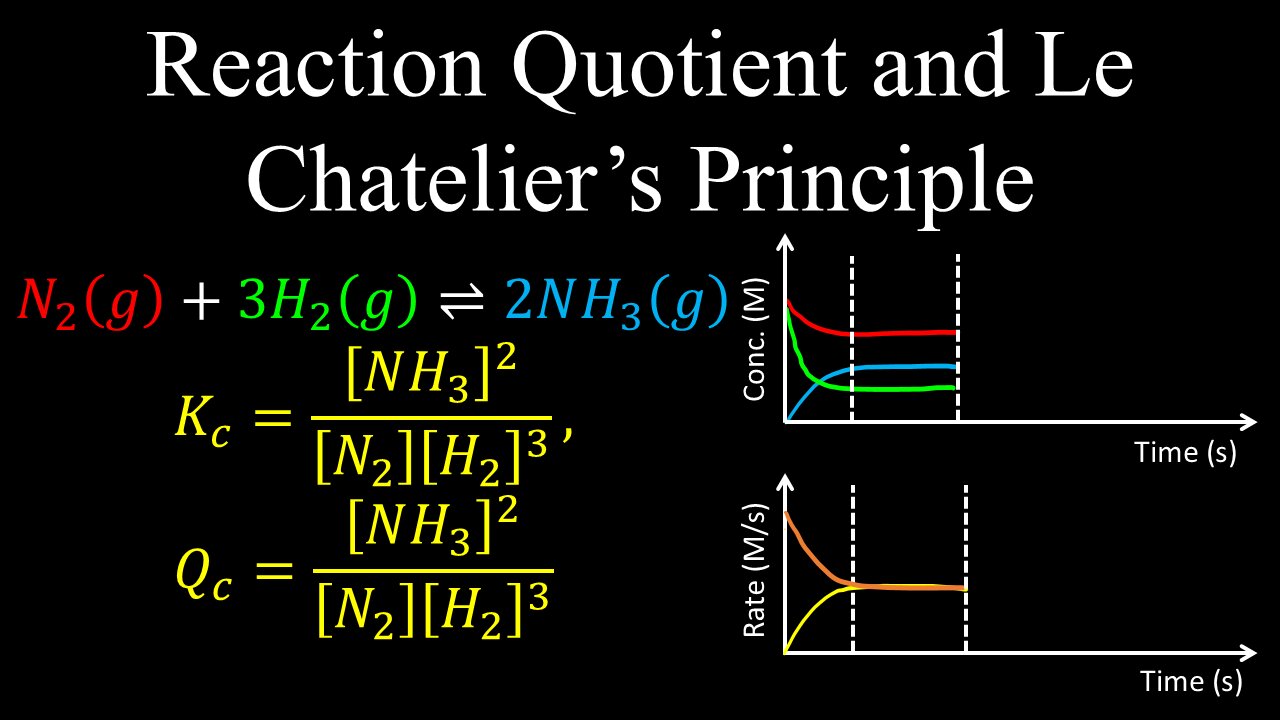
Reaction Quotient and Le Chatelier's Principle, Equilibrium, Example - Chemistry
2 months ago
105
Science
Education
reaction quotient
le chatelier's principle
chemistry
equilibrium
equilibrium constant
le chateliers principle equilibrium
chemical equilibrium
le chateliers principle
general chemistry
This video explains the meaning of reaction quotient vs equilibrium constant, including definition, what happens if Q is bigger or less than K, and the difference between Q and K. You will understand how different stresses like concentration, temperature and catalysts affect the concentrations of reactants and products, the the rate of the forward and backward reaction.
0:00 Reaction quotient, effect of concentration
2:22 Reaction quotient, effect of temperature
4:18 Worked example
Loading comments...
-
 1:28:42
1:28:42
Redacted News
5 hours agoBREAKING! SOMETHING BIG IS HAPPENING IN EUROPE ALL OUT WAR IS COMING AGAINST RUSSIA, TRUMP FURIOUS
108K252 -
 47:50
47:50
Candace Show Podcast
5 hours agoBREAKING: Judge Makes Statement Regarding Taylor Swift's Text Messages. | Candace Ep 155
94.3K100 -
 LIVE
LIVE
Josh Pate's College Football Show
2 hours agoCFB’s Most Hated Teams | FSU & Clemson Future | Big Ten Win Totals | Star Rankings Overrated?
91 watching -
 1:33:47
1:33:47
CatfishedOnline
4 hours agoGoing Live With Robert - Weekly Recap
19.4K -
 55:18
55:18
LFA TV
1 day agoEurope’s Sudden Turn Against America | TRUMPET DAILY 3.6.25 7PM
25.5K3 -
 4:21
4:21
Tundra Tactical
3 hours ago $1.38 earnedPam Bondi MUST Enforce Due Process NOW!
18.4K1 -
 56:42
56:42
VSiNLive
4 hours agoFollow the Money with Mitch Moss & Pauly Howard | Hour 1
43.6K1 -
 1:05:32
1:05:32
In The Litter Box w/ Jewels & Catturd
1 day agoShalom Hamas | In the Litter Box w/ Jewels & Catturd – Ep. 756 – 3/6/2025
98.7K37 -
 1:23:00
1:23:00
Sean Unpaved
6 hours ago $2.98 earnedNFL Free Agency
50.9K3 -
 18:25
18:25
Stephen Gardner
5 hours ago🔥The REAL REASON the Epstein Files are being HIDDEN | I CONFRONT Alan Dershowitz for details!
63.3K103