Premium Only Content
New experimental mRNA trial suspended
Vaccines and Related Biological Products Advisory Committee December 12, 2024 Meeting Announcement
Entire briefing document
https://www.fda.gov/media/184301/download
Pediatric RSV vaccine trial enrollment on hold in US, VRBPAC says
The halt follows a severe respiratory disease safety signal observed in a July 2024 phase 1 trial of Moderna's mRNA-1345 and mRNA-1365 vaccine candidates.
A phase I trial
The effects of the medication on about 20 to 80 healthy volunteers
To evaluating safety and ideal dosage
70% move on to phase II
This study, safety, tolerability, and immunogenicity
Who were the subjects
Children aged younger than 2 years
Respiratory syncytial virus (RSV) -naïve children aged 2 through 5 years
The halt of this mRNA RSV vaccine
Severe respiratory disease safety signal
Safety signal led to study pause
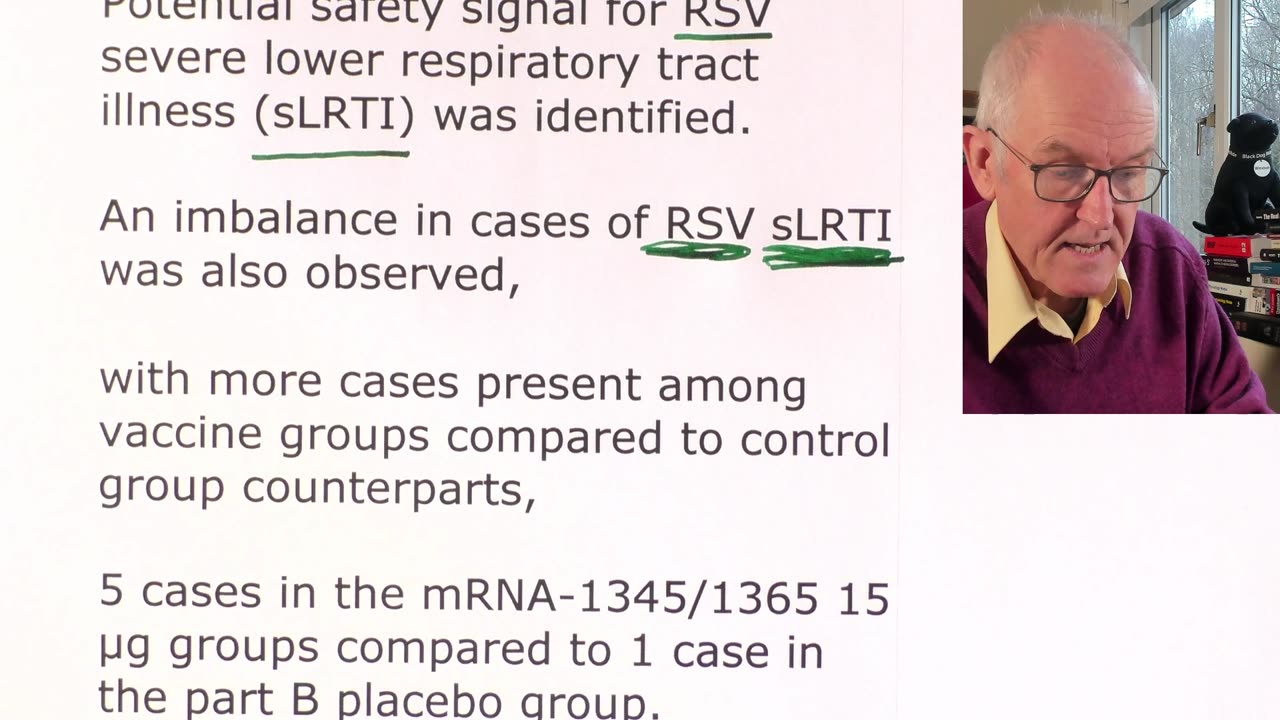
Potential safety signal for RSV severe lower respiratory tract illness (sLRTI) was identified.
An imbalance in cases of RSV sLRTI was also observed,
with more cases present among vaccine groups compared to control group counterparts,
5 cases in the mRNA-1345/1365 15 µg groups compared to 1 case in the part B placebo group.
Of these 6 cases, 5 required hospitalization, including 1 infant who required mechanical ventilation.
which raised concern for vaccine-associated enhanced respiratory disease.
The protocol’s study pause criterion of any sLRTI with positive polymerase chain reaction (PCR) for RSV in ≥2 participants was met.
One, two and three dose parts planned
Part A (Cohorts 1 and 2)
30 µg mRNA-1345, 30 µg mRNA-1365, and placebo
approximately 90 participants 8 months to <24 months of age
(randomized in a 1:1:1 ratio, respectively)."
"Part B (Cohorts 3 through 6)
2 dose levels of mRNA-1345 and mRNA-1365 and placebo
Approximately 120 participants 5 months to <8 months of age
(randomized in a 1:1:1 ratio, respectively)
Part C (Cohorts 7 and 8)
3 doses of 30 µg mRNA-1345
Approximately 100 participants 8 months to <12 months of age
who have (Cohort 7) or have not (Cohort 8) previously received nirsevimab.
VRBPAC meeting and outlook
Vaccines and Related Biological Products Advisory Committee
Vaccine-associated enhanced respiratory disease
Currently considering criteria for recommencement
Scam Accounts
https://www.facebook.com/Campbellteaching/
https://www.facebook.com/dr.john.campbell/
Genuine Campbell Teaching Accounts
https://substack.com/@johninengland?utm_source=user-menu
https://www.paypal.com/mep/dashboard
https://www.paypal.com/donate/?cmd=_s-xclick&hosted_button_id=78GGHGLK5ZXAE
-
 16:47
16:47
Dr. John Campbell
30 days agoAutism and drones
21.4K169 -
 LIVE
LIVE
Scammer Payback
57 minutes agoCalling Scammers Live
386 watching -
 LIVE
LIVE
Stephen Gardner
43 minutes ago🔥Congress DROPS Bad News on Newsom and Biden!
915 watching -
 LIVE
LIVE
Twins Pod
43 minutes agoHe Left The RAP Industry To Make Christian Music! | Twins Pod - Episode 48 - Bryson Gray
1,097 watching -
 1:30:05
1:30:05
The Quartering
2 hours agoSupreme Court RULES On TikTok Ban, Kamala Harris At Rock Bottom & Brawls Break Out At Costco!
22K6 -
 1:36:11
1:36:11
Tucker Carlson
2 hours agoSean Davis: Trump Shooting Update, & the Real Reason Congress Refuses to Investigate
83.5K61 -
 2:55:32
2:55:32
The Dana Show with Dana Loesch
3 hours agoTHE END OF TIKTOK | The Dana Show LIVE On Rumble!
2.39K3 -
 1:22:57
1:22:57
The Criminal Connection Podcast
3 hours agoPADDY DOHERTY: Dougie Joyce RESPONSE! Bare Knuckle Fighting, Sausage Fests & Assassination Attempts
8 -
 1:57:23
1:57:23
The Charlie Kirk Show
3 hours agoConfirmation Mania: Day 4 + AMA | Comer | 1.17.2025
115K42 -
 LIVE
LIVE
SIEFE
3 hours agoRED DEAD REDEMPTION 2 LIVE!
107 watching