Premium Only Content
MCAT General Chemistry Lectures Review Prep Part 2 – Equations & Practice Questions
This mcat review prep video tutorial focuses on the general chemistry section of the mcat. This is the part 2 version and it contains plenty of notes, concepts, equations, formulas, example problems and practice questions. Here is a list of topics included in this video:
MCAT General Chemistry Part 1 - 8.5 Hour Review:
https://bit.ly/3xEWUuI
MCAT General Chemistry Part 2 - 7.5 Hour Review:
https://bit.ly/4awPGaI
MCAT Organic Chemistry Reactions Part 1 - 4 Hour Review:
https://bit.ly/43WXZuf
MCAT Organic Chemistry Reactions Part 2 - 5 Hour Review:
https://bit.ly/3TQXzkp
1. State Functions – Enthalpy, Entropy, and Free Energy
2. Internal Energy of a System – Heat Transfer and Work
3. System vs Surroundings – Endothermic and Exothermic Processes
4. Pressure, Volume and Work – Gas Expansion vs Compression
5. Temperature Change vs Phase Change – Heat Transfer Problems
6. Heat of Vaporization and Enthalpy of Fusion
7. Freezing, Melting, Vaporization, Condensation, Sublimation and Deposition
8. Heats of Formation, Enthalpy of Reaction, Hess Law, Calorimetry, and Bond Dissociation Energy
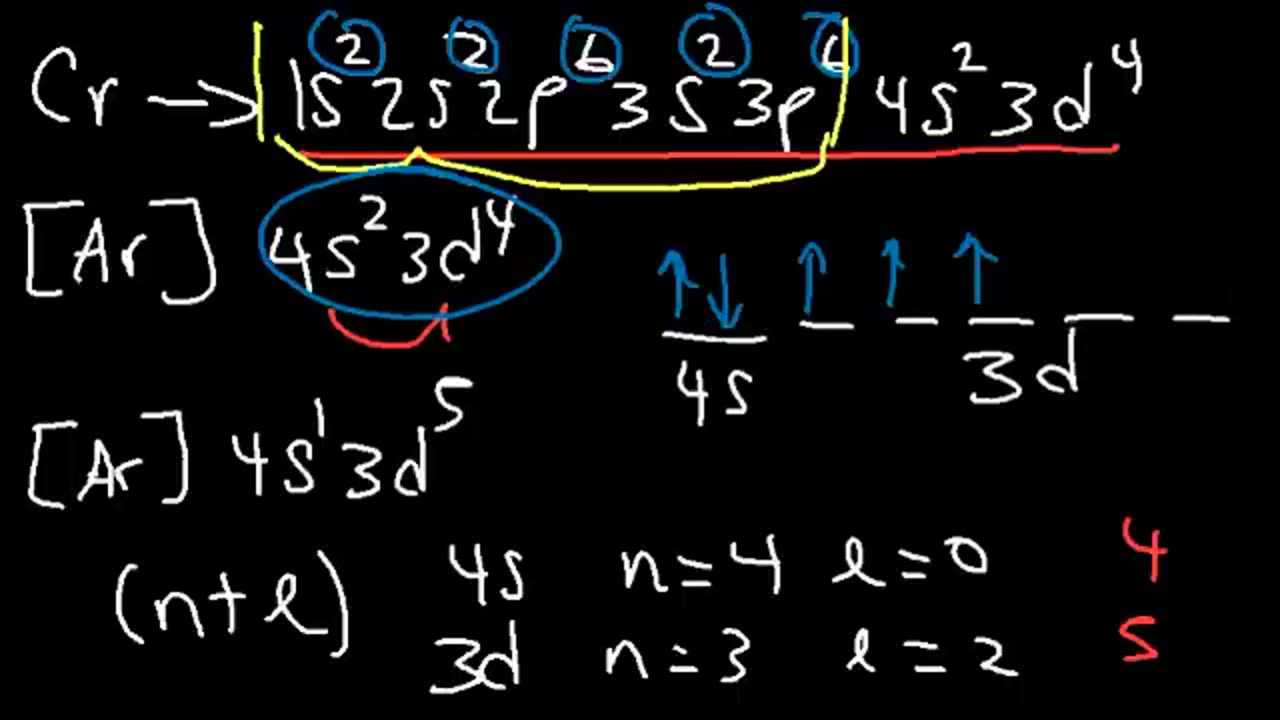
9. Quantum Mechanics – n, l, ml, and ms. S, P, D, F orbitals. Energy Levels and Sublevels.
10. Ground State Electron Configuration vs Noble Gas Notation With Orbital Diagrams
12. Radio Waves, Microwaves, Infrared, Visible Light, Ultraviolet, X-Rays, and Gamma Rays
13. Electromagnetic Radiation – Frequency, Wavelength, Energy of a Photon, and Speed of Light
14. Ionization Energy, Electron Affinity, Atomic Size, Ionic Radii, Metallic Character, & Electronegativity
15. Lewis Structures, Hybridization, Molecular Geometry, Bond Angle, Polar and Nonpolar
16. Formal Charge Calculations, Bond Order, Sigma and Pi Bonds, Bond Length vs Strength
17. Intermolecular Forces – Ion Dipole, Hydrogen Bonds, Dipole Dipole and London Dispersion Forces
18. Boiling Point vs Vapor Pressure – Molar Mass, Polarizability, and Temporary Induced Dipoles
19. Classius Clayperon Equation
20. Vapor Pressure and Temperature, Boiling Point vs Elevation
21. Molality, Molarity, Mass %, and Density Calculations – Solute, Solvent, and Solutions
22. Henry’s Law – Gas Solubility vs Pressure vs Temperature
23. Colligative Properties – Boiling Point Elevation, Freezing Point Depression
24. Vapor Pressure Lowering – Raoult’s Law and Osmotic Pressure
25. Phase Diagrams – Critical Point, Triple Point, Normal Melting Point, and Normal Boiling Point
-
 13:10
13:10
TheOrganicChemistryTutor
3 days agoDependent and Independent Variables
44 -
 3:14:33
3:14:33
Joe Donuts Gaming
5 hours ago🟢 Live : Christmas is Here!! | Fortnite, Caroling, Light Tours and Donos !!
17K7 -
 LIVE
LIVE
CLUJ
4 hours agoCHRISTMAS EVENING HYPE!! LETS HAVE FUN GAMING!!
901 watching -
![I AM FINALLY BACK :: PUBG: BATTLEGROUNDS :: RUMBLE NOW HAS GIFTED SUBS!!! [Merry Christmas] {18+}](https://1a-1791.com/video/fwe1/22/s8/1/e/f/C/6/efC6v.0kob-small-I-AM-FINALLY-BACK-PUBG-BATT.jpg) LIVE
LIVE
a12cat34dog
6 hours agoI AM FINALLY BACK :: PUBG: BATTLEGROUNDS :: RUMBLE NOW HAS GIFTED SUBS!!! [Merry Christmas] {18+}
128 watching -
 3:55:42
3:55:42
STARM1X16
5 hours agoMerry Christmas Fortnite
22.7K3 -
 2:45:33
2:45:33
Sgtfinesse
5 hours agoMerry Christmas Night
30.5K15 -
 LIVE
LIVE
tacetmort3m
22 hours ago🔴 LIVE - (MERRY CHRISTMAS) TIME TO SPREAD DEMOCRACY - HELLDIVERS 2 OMENS OF TYRANNY
80 watching -
 12:42
12:42
Cooking with Gruel
20 hours agoBrown Butter Trifle with Salted Caramel and Cinnamon Apple
10.1K3 -
 2:46
2:46
BIG NEM
8 hours agoDiscovering RAKIJA: The Holy Liquer of the Balkans
8.05K2 -
 1:11:38
1:11:38
Film Threat
13 hours agoCHRISTMAS DAY CHILL STREAM WITH CHRIS GORE | Hollywood on the Rocks
131K24