Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
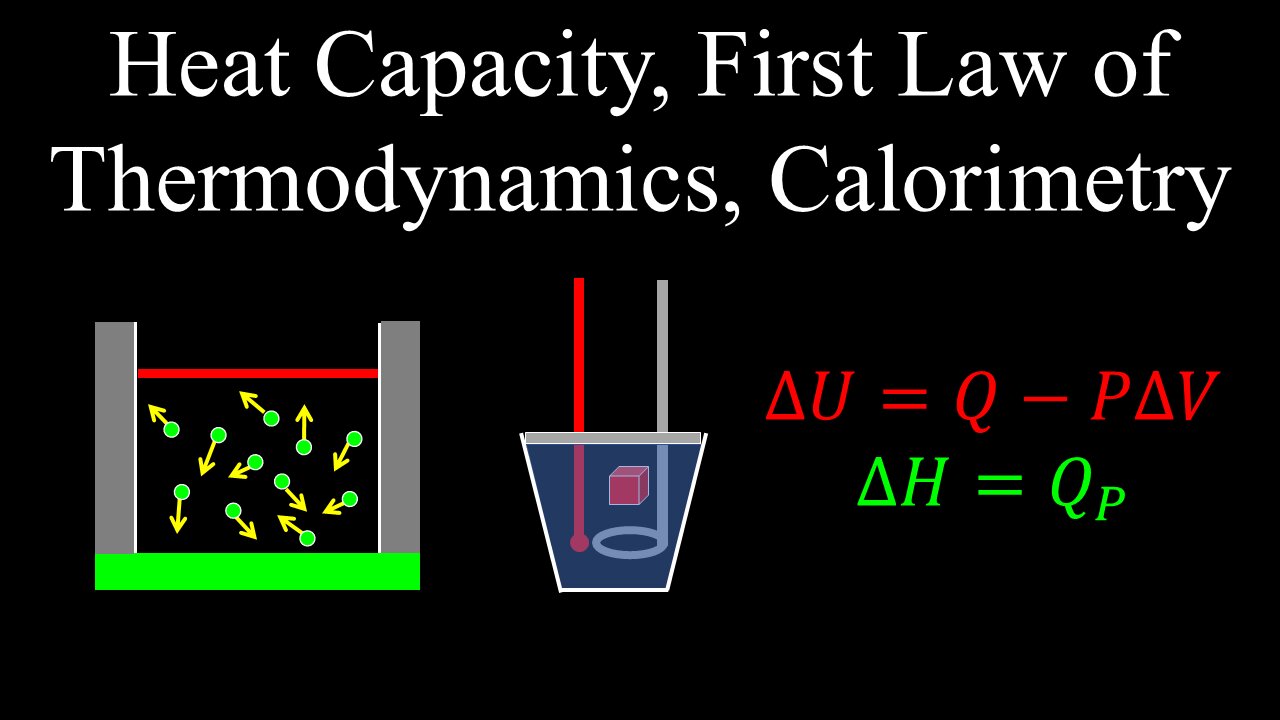
Heat Capacity, First Law of Thermodynamics, Enthalpy, State Variables, Calorimetry - Chemistry
1 month ago
62
Science
Education
first law of thermodynamics
thermodynamics
specific heat capacity chemistry
the first law of thermodynamics
first law of thermodynamics in physics
state variables
chemistry
calorimetry
heat capacity in thermodynamics
Basic introduction into the first law of thermodynamics, an example that highlights its significance, its application in chemistry, basic equation/formula, why it is used, what it deals with. Change in enthalpy or heat content is explained in simple terms, with a basic definition and symbol. The concept of calorimetry, how heat is measured in a calorimeter, basic experiment and analysis using heat capacity and specific heat and what is meant by it.
0:00 Heat capacity
3:27 First law of thermodynamics
5:29 Enthalpy, state variables
7:40 Constant pressure calorimetry
10:03 Worked example
Loading comments...
-
 16:49
16:49
Dave Portnoy
2 hours agoDavey Day Trader Presented by Kraken - January 3, 2025
11.3K3 -
![🔴[LIVE TRADING] Tesla Falls, Nvidia Bounces & Payday Friday || The MK Show](https://1a-1791.com/video/fwe2/56/s8/1/d/a/4/k/da4kw.0kob-small-The-MK-Show-Jan.-3rd.jpg) LIVE
LIVE
Matt Kohrs
7 hours ago🔴[LIVE TRADING] Tesla Falls, Nvidia Bounces & Payday Friday || The MK Show
1,219 watching -
 1:33:19
1:33:19
Tactical Advisor
2 hours agoThe Vault Room Podcast 007 | Terrorist Attacks Update
13.1K1 -
 1:45:30
1:45:30
Game On!
11 hours ago $1.14 earnedNotre Dame proves that the SEC is the WORST conference in college football!
11.9K4 -
 1:49:52
1:49:52
Jeff Ahern
2 hours ago $1.34 earnedFriday Freak out with Jeff Ahern ( The Dr Michael Schwartz interview)
14K2 -
 15:04
15:04
Misha Petrov
21 hours agoThese Leftist Tattoos Are UNHINGED!
24.7K79 -
 8:27
8:27
Dr. Nick Zyrowski
1 day agoWhat to Eat After Fasting - This Diet Heals You!
50.9K13 -
 15:15
15:15
Chris From The 740
14 hours ago $1.77 earnedThe C&H Precision Comp Is The SRO Alternative You've Been Waiting For
29K7 -
 24:02
24:02
Bek Lover Podcast
1 day agoAmerica Under Attack - Danger In Every State?
23.6K15 -
 1:03:40
1:03:40
Uncommon Sense In Current Times
1 day ago $1.44 earned"Bar Ministry: Reaching the Lost in Unlikely Places with Randall Reeder"
33.6K5