Premium Only Content
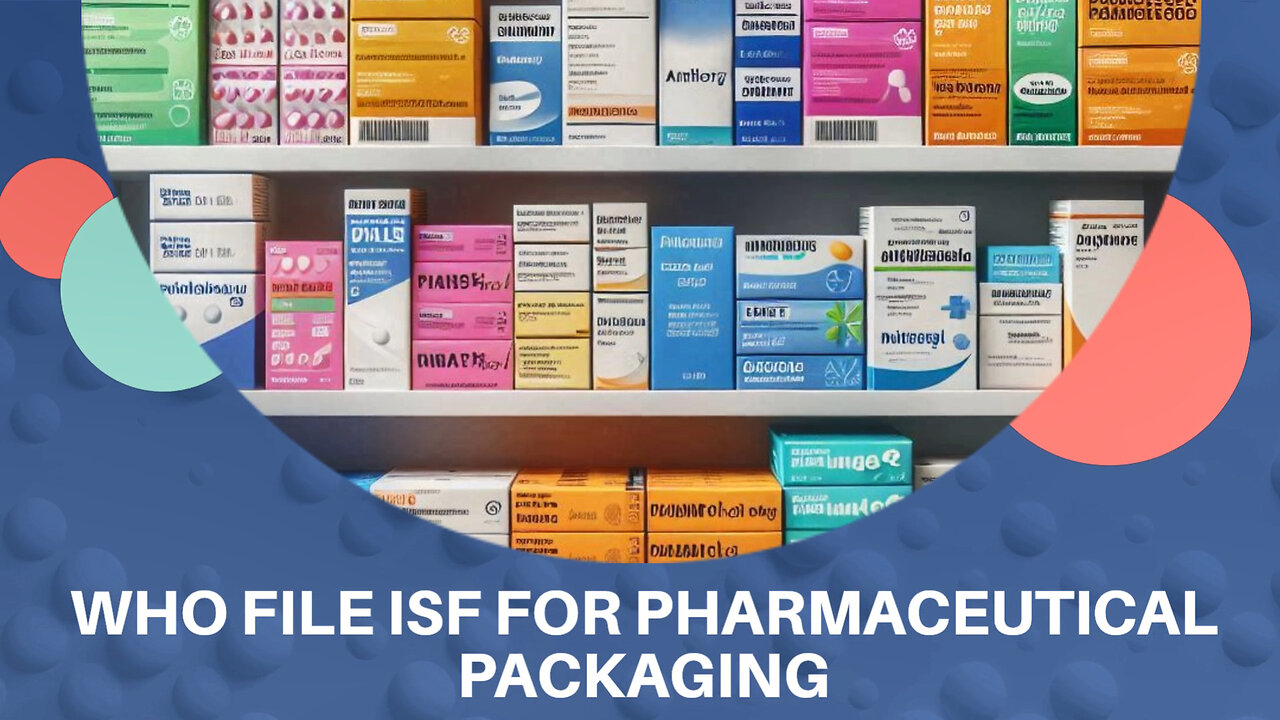
Navigating ISF: Essential Tips for Pharmaceutical Importers
* e-Customs Clearing
* 714-855-3556
* info@eCustomsClearing.com
* https://ecustomsclearing.com
SUBSCRIBE → https://www.youtube.com/channel/UCUwQ3bbxUUhIxDkV304ipNQ?sub_confirmation=1
Importer Security Filing (ISF) is vital in international trade, especially for businesses in pharmaceutical packaging, as it provides information required by US Customs and Border Protection to enhance cargo security. The responsibility to file ISF primarily lies with the importer of record, who may engage customs brokers to assist in the process. Compliance with ISF requirements is crucial due to the stringent regulations governing pharmaceutical packaging, given the impact on public health. Accurate ISF filings are key in preventing border delays and ensuring timely delivery of critical pharmaceutical products.
Customs brokers play a significant role in facilitating ISF filings for importers, leveraging expertise in customs regulations to ensure compliance and seamless import processes. For pharmaceutical packaging, precise reporting of information such as supplier details, cargo description, and product origins is essential to meet regulatory standards and expedite customs clearance. Timely submission of ISF filings, at least 24 hours before goods are loaded for the US, is critical in allowing Customs and Border Protection to assess cargo risks effectively, particularly for urgent medical supplies.
Failure to comply with ISF filing requirements can lead to penalties and clearance delays, posing risks for pharmaceutical importers and impacting supply chains and patient access to essential products. Technology advancements have transformed ISF filing processes, enabling real-time tracking and accurate submissions through customs broker software. Customs bonds serve as financial guarantees for duties and penalties, offering protection for pharmaceutical importers against unforeseen customs issues.
To optimize ISF filings, importers in pharmaceutical packaging should maintain comprehensive shipment records, collaborate closely with customs brokers for regulatory guidance, and train staff on ISF requirements to enhance compliance and operational efficiency. Future trends in ISF and customs brokerage are expected to move towards increased digitization, requiring businesses to adapt swiftly to evolving standards for continued compliance and industry leadership. Understanding the complexities of ISF and customs brokerage is essential for importers in the pharmaceutical sector to navigate international trade effectively and ensure timely access to critical pharmaceuticals for public health.
#usimportbond #isfcustomsbroker #uscustomsclearing #isfentry
Video Disclaimer Here: This educational video is not linked to any US government entity.
0:34 - Key Players in Filing the ISF
1:03 - Specifics of Pharmaceutical Packaging
1:29 - Information Required for ISF
-
 3:21:29
3:21:29
Alex Zedra
9 hours agoLIVE! New Game | R.E.P.O
42.4K1 -
 1:52:42
1:52:42
Kim Iversen
11 hours ago💰 CHA-CHING! 💰 Trump Unveils Big Money Plans For Gaza AND America
57.9K172 -
 1:05:28
1:05:28
Flyover Conservatives
23 hours agoUkraine’s Dirty Secret: The Christian Persecution No One Wants to Talk About - Alex Newman | FOC Show
58.5K19 -
 2:00:20
2:00:20
Glenn Greenwald
15 hours agoThe View from Moscow: Key Russian Analyst Aleksandr Dugin on Trump, Ukraine, Russia, and Globalism | SYSTEM UPDATE #414
141K64 -
 1:10:55
1:10:55
Donald Trump Jr.
13 hours agoBREAKING NEWS: My Father Revokes Biden-Maduro Oil License, LIVE with Maria Corina Machado | Triggered Ep.220
210K196 -
 1:25:29
1:25:29
Sarah Westall
11 hours agoX-Files True History, Project Blue Beam, Cabal Faction War w/ Former FBI Agent John DeSouza
88.7K16 -
 7:03:49
7:03:49
Dr Disrespect
18 hours ago🔴LIVE - DR DISRESPECT - NEW PC VS. DELTA FORCE - MAX SETTINGS
175K27 -
 49:04
49:04
Lights, Camera, Barstool
1 day agoIs The Monkey The Worst Movie Of The Year?? + Amazon Gets Bond
78K4 -
 24:19
24:19
Adam Carolla
1 day agoDiddy’s Legal Drama Escalates, Smuggler Caught Hiding WHAT? + Philly Eagles & The White House #news
145K21 -
 10:12
10:12
Mike Rowe
2 days agoClint Hill: What A Man. What A Life. | The Way I Heard It with Mike Rowe
136K17