Premium Only Content
Aaron Siri about vaccine package inserts
Aaron Siri: "Going back to the 2012 IOM report we talked about earlier, so I noted to you then that the CDC and HRC paid the IOM to review what they said were the 158 most commonly claimed serious injuries from vaccines. Now these are the ones that they say the most commonly claimed injuries.
In that review, the IOM found that for 18 of them, evidence supported a causal relationship. These are serious reactions. Okay. So they said, yeah, vaccines cause 18, which is troubling. Five of the issues they rejected, including MMR and autism, was one of the five. But this maybe is the most troubling. For 135 of the most commonly claimed serious injuries from vaccines by their admission, the evidence was insufficient to reach a conclusion.
They hadn't done the studies. They don't know because their clinical trial data is vacuous as we've seen and they just don't do the studies otherwise for the most part just like autism. There's a link to the Institute of Medicine report. You can see it directly and I would focus on the uh uh on the there's a summary table where you can look at all of the conditions by vaccine and what the cause of the conclusion is. It shows again, they're not studying even the most commonly claimed injuries.
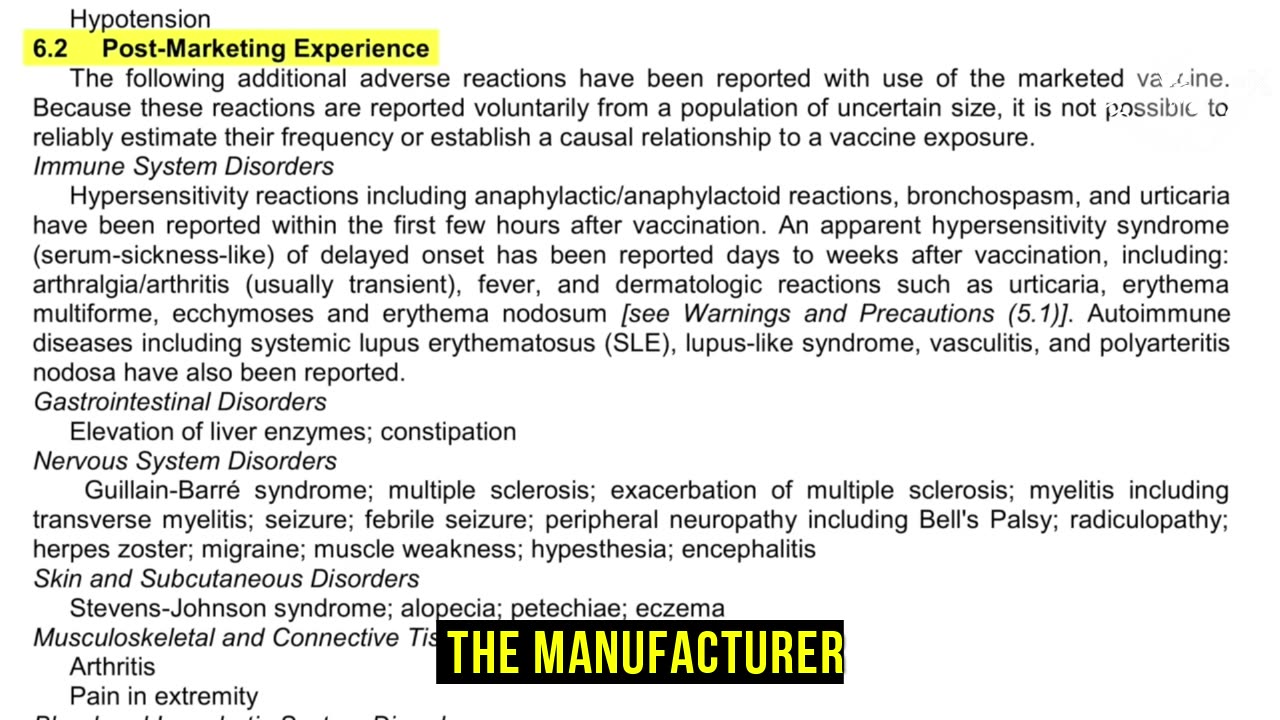
What injuries can vaccines cause? Okay. Now in the package insert, we looked at section 6.1 before, remember, and it, that showed the clinical trial experience. Well, in section 6.2 is the post marketing experience. Okay. And that is after it's licensed, the manufacturer, if they have a believes in to believe there's a causal, not correlation, causal relationship between the vaccine and the injury.
They're supposed to put it in section 6.2. They're not allowed to put stuff in that's just correlation. How do I know that? I'll read you the federal regulation. This is the code of federal regulation, section 21 CFR 2.1.57. And it provides that package interest of vaccine should include quote, only, only those adverse events for which there is some basically there's a causal relationship between the drug and occurrence of the adverse event. Not correlation, causal relationship. And I can tell you in practice, in practice from my experience, and you can go and look at the licensure documents and so forth, they really only put stuff in vaccine package inserts where they really see a causal relationship because under the 1986 act for the most part, you can still get them for fraud. So if they have, they know it can cause and they don't put it in there, you can potentially sue them.
But otherwise, they're not really concerned about putting it in there."
-
 1:08:09
1:08:09
Donald Trump Jr.
16 hours agoFBI Dream Team, Plus Taking Your Questions Live! | Triggered Ep.219
213K285 -
 7:32:37
7:32:37
Akademiks
15 hours agoDrake and PartyNextDoor '$$$4U' Album Sells 250K first week. BIG AK IS BACK.
128K19 -
 3:12:08
3:12:08
MyronGainesX
14 hours ago $33.46 earnedDan Bongino Named FBI Deputy Director, Trump Meets Macron, And More!
103K32 -
 3:12:31
3:12:31
vivafrei
14 hours agoBarnes Live from Seattle - Defending Benshoof in a Case that is CRAY CRAY!
177K54 -
 2:12:12
2:12:12
Robert Gouveia
14 hours agoLiberals EXPLODE over Elon's Email; Lawsuits FLY; Sanctions?? Congrats Dan!
134K79 -
 1:33:36
1:33:36
Redacted News
14 hours agoBREAKING! PUTIN LAUNCHES MASSIVE OFFENSIVE IN UKRAINE AS EUROPEAN LEADERS PUSH FOR MORE WAR
207K280 -
 44:39
44:39
Kimberly Guilfoyle
15 hours agoBetter Days Ahead for the FBI, Live with Asm Bill Essayli & John Koufos | Ep.199
127K34 -
 1:40:29
1:40:29
In The Litter Box w/ Jewels & Catturd
1 day agoWhat Did You Do Last Week? | In the Litter Box w/ Jewels & Catturd – Ep. 748 – 2/24/2025
164K70 -
 23:34
23:34
Stephen Gardner
15 hours ago🔥CNN PANICS over $5000 DOGE Dividend | Trump Orders bigger Audits
103K173 -
 1:53:54
1:53:54
The White House
17 hours agoPresident Trump Holds a Press Conference with President Emmanuel Macron of France
112K129