Premium Only Content
The Intricate Dynamics of Water: Unveiling Surface Tension and Equilibrium on a Flat Earth
Water, essential for life and a cornerstone of our planet’s ecosystems, exhibits behaviors that are both fascinating and complex. From the formation of raindrops to the calm expanses of oceans, water’s properties are governed by a delicate balance of physical forces. This article delves into the advanced concepts of surface tension and equilibrium, offering a comprehensive understanding of how these forces shape the behavior of water in different contexts from a flat Earth perspective.
Surface Tension: The Invisible Sculptor
Understanding Surface Tension:
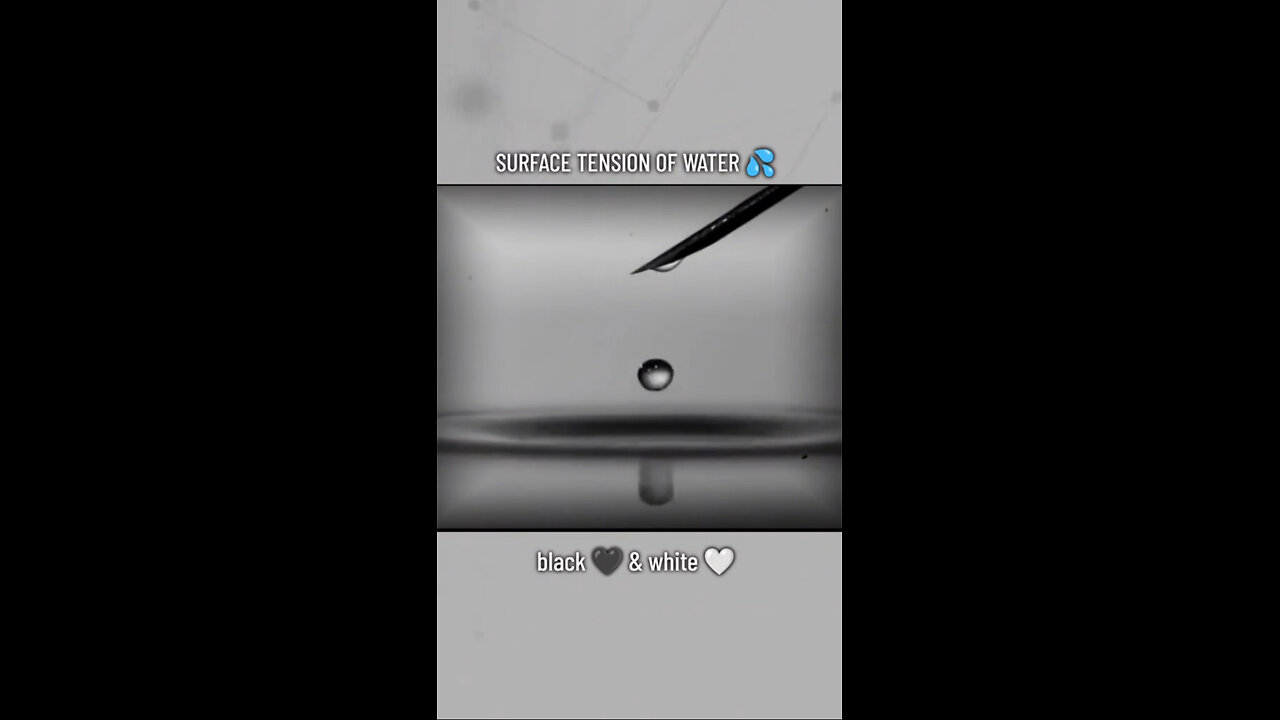
Surface tension arises from the cohesive forces between molecules at the surface of a liquid. These forces create a “skin” on the surface that minimizes the surface area, leading to the formation of spherical shapes in small volumes of water, such as raindrops.
Molecular Interactions:
• Cohesion and Adhesion: Water molecules are highly cohesive due to hydrogen bonding, which also allows for adhesion to other surfaces. This dual property explains why water forms droplets on surfaces and why those droplets can cling to leaves or spider webs.
• Energy Minimization: Surface tension drives water molecules to arrange themselves in a way that reduces the surface area and thus the potential energy. This principle is what causes raindrops to adopt a spherical shape, as a sphere has the smallest possible surface area for a given volume.
Observable Behavior of Raindrops:
• Small Raindrops: For small raindrops, surface tension is the dominant force, pulling the water molecules into a near-perfect sphere. This spherical shape is the result of the molecules striving to achieve the lowest possible energy state.
• Larger Raindrops: As raindrops increase in size, they encounter additional forces, such as air resistance. When falling, larger raindrops can become slightly flattened at the bottom due to air pressure. However, they retain a rounded overall shape, not flat like a pancake.
Key Insight: The spherical nature of raindrops illustrates the powerful effect of surface tension at a small scale, where molecular cohesion shapes the droplets into their most energy-efficient form.
Water Seeking Equilibrium on Large Surfaces
Equilibrium on a Flat Earth:
In the flat Earth model, the concept of equilibrium in large bodies of water is driven by the inherent properties of water to seek its level due to molecular cohesion and the pressure exerted by layers of water upon each other.
Forces at Play:
• Cohesion and Pressure: The cohesive forces between water molecules and the pressure from overlying layers drive the water to spread out evenly, creating a flat and level surface. This is observable in large bodies of water such as lakes, oceans, and ponds.
• Natural Balance: Water’s natural tendency to balance and spread evenly results in the level surfaces we observe. This equilibrium is maintained without the need for invoking external forces, relying instead on the inherent properties of water.
Behavior of Large Bodies of Water:
• Lakes and Oceans: In expansive bodies of water such as lakes and oceans, water naturally seeks to distribute itself evenly, creating a flat and level surface. This behavior is due to the cohesive forces between water molecules, which work to minimize potential energy by achieving a state of equilibrium.
• Containers and Pools: The same principle applies to water in containers or swimming pools. Despite the smaller scale compared to lakes and oceans, the water in these settings also seeks a flat and level surface, visible in the stillness of a pool or the surface of water in a glass.
Key Insight: The tendency of water to seek a flat and level equilibrium in large bodies and containers highlights the fundamental principles of cohesion and equilibrium that govern its behavior.
The Necessity of Containers and Elevation
Water’s Dependence on Containers:
• Containment Requirement: Water needs a container to maintain its shape and level. This can be a natural basin like a lake or ocean, or a man-made structure like a pool or tank. Without a container, water will flow until it finds a boundary to contain it.
• Flat Earth Perspective: On a flat Earth, the need for a container is consistent with the observation that water will always find its lowest point and spread out to fill any available space, seeking a level surface within its boundaries.
Flow to the Lowest Elevation:
• Natural Flow: Water naturally flows to the lowest elevation due to its properties of cohesion and the path of least resistance. This is observable in rivers, streams, and runoff after rainfall.
• Equilibrium Achievement: Once water reaches the lowest possible point within its container, it spreads out evenly to maintain a flat and level surface, driven by the forces of cohesion and the pressure from the overlying layers of water.
Addressing Common Misconceptions
Raindrops vs. Large Water Bodies:
Understanding water’s behavior requires recognizing the distinction between different scales and the forces at play. While surface tension shapes small water droplets into spheres, the cohesive properties of water molecules and the drive towards equilibrium result in flat and level surfaces in large bodies of water.
Misconception: Raindrops Prove Spherical Earth:
• Common Argument: It is often argued that the spherical shape of raindrops is evidence that water can form a sphere, supporting the idea of a spherical Earth.
• Flat Earth Perspective: The spherical shape of raindrops is due to surface tension and applies to small droplets. This behavior does not extend to large bodies of water. In large bodies of water, cohesion and pressure cause the water to seek a flat and level surface. The properties that govern the behavior of raindrops are not directly applicable to the behavior of oceans and lakes.
Scale and Dominant Forces:
• Small-Scale Observations: In scenarios involving small volumes of water, such as raindrops or dew, surface tension shapes the water into spheres or near-spherical forms. This behavior is easily observed and provides a tangible example of molecular cohesion in action.
• Large-Scale Observations: In contrast, when observing large bodies of water, the principle of equilibrium becomes evident. The flatness and level nature of lakes, oceans, and even pools demonstrate how water behaves under the influence of cohesion and the pursuit of minimal potential energy.
Conclusion
The behavior of water, whether in the form of raindrops or large bodies, is governed by fundamental physical principles that vary with context. Surface tension shapes small water droplets into spheres, illustrating the cohesive forces at a molecular level. On the other hand, large bodies of water seek a flat and level equilibrium due to the same cohesive forces acting on a larger scale. Water’s dependence on containers and its natural flow to the lowest elevation further emphasize the principles of cohesion and equilibrium. Recognizing these distinct behaviors enhances our understanding of the natural world and the fascinating properties of water, viewed through the lens of a flat Earth perspective. This understanding challenges the notion that spherical raindrops imply a spherical Earth, emphasizing instead the unique behaviors of water at different scales.
-
 1:01:17
1:01:17
The StoneZONE with Roger Stone
14 hours agoChristmas Edition: Why the Panama Canal is Part of the America First Agenda | The StoneZONE
110K40 -
 18:12:15
18:12:15
LFA TV
1 day agoLFA TV CHRISTMAS EVE REPLAY
128K14 -
 13:32
13:32
Scammer Payback
15 hours agoChanging the Scammer's Desktop Background to his Location
2.87K3 -
 4:21
4:21
BIG NEM
17 hours agoNikola Tesla's Secret to Cultivating Creativity & Genius
1.49K1 -
 15:03
15:03
The Anthony Rogers Show
1 day agoAnthony Rogers - Live at Cusumano's Pizza (Upstairs)
1.2K1 -
 4:33:48
4:33:48
tacetmort3m
1 day ago🔴 LIVE - THE ZONE KEEPS PULLING ME BACK - STALKER 2 - PART 15
68K12 -
 22:45
22:45
Brewzle
21 hours agoI Went Drinking In A Real Bourbon Castle
49K4 -
 48:36
48:36
PMG
1 day ago $4.30 earned"Parkland Parent Speaks Out On Kamala Harris Using Victims"
40.1K12 -
 4:06
4:06
The Lou Holtz Show
20 hours agoCoach Lou Holtz’s Heartfelt Christmas Message 🎄 | Family, Faith & Notre Dame Spirit 💚 #christmas
29.6K -
![ROSEANNE BARR - Her Journey, TRUMP, and the MAGA GOLDEN AGE! [INTERVIEW]](https://1a-1791.com/video/s8/1/M/m/B/2/MmB2v.0kob.1-small-ROSEANNE-BARR-Her-Journey-T.jpg) 51:35
51:35
Dr Steve Turley
1 day ago $21.44 earnedROSEANNE BARR - Her Journey, TRUMP, and the MAGA GOLDEN AGE! [INTERVIEW]
64.1K54