Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
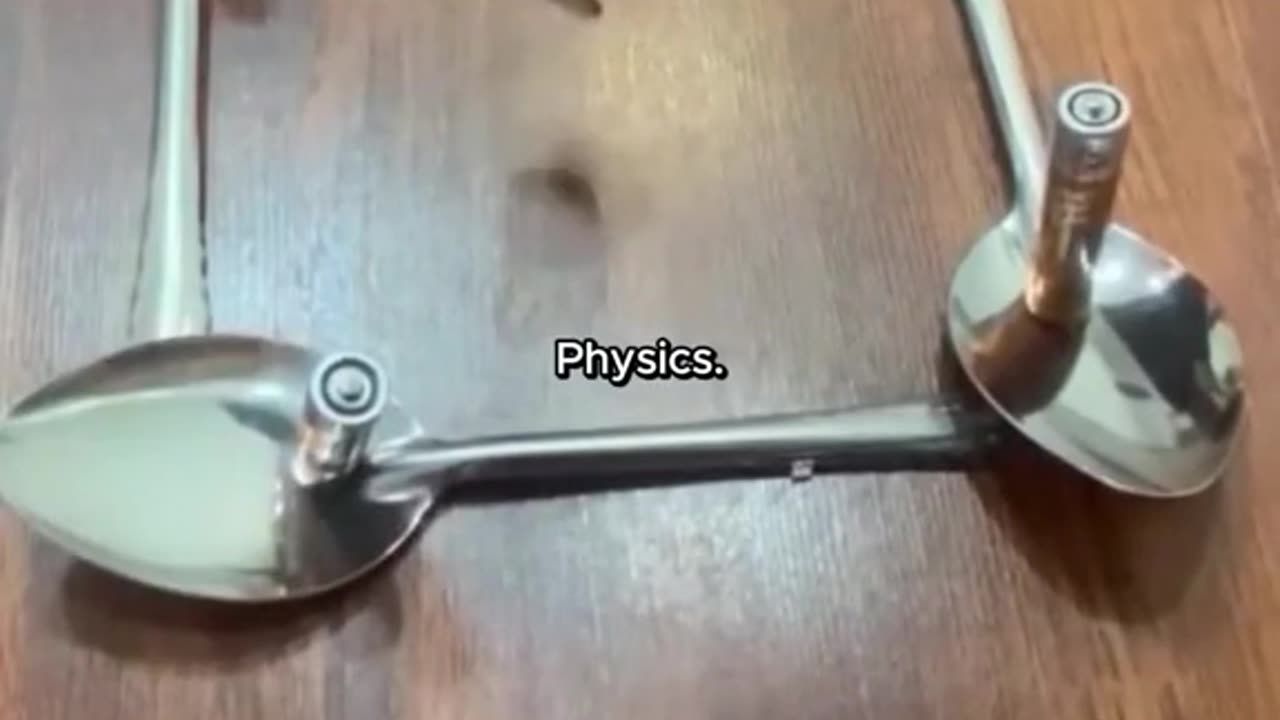
Physics experiment
11 months ago
11
Water molecules are polar, with oxygen having a slight negative charge and hydrogen having a slight positive charge due to electrostatic forces. This polarity leads to hydrogen bonding between water molecules, resulting in high surface tension and cohesion. Electrostatic interactions between water molecules also contribute to its ability to dissolve many substances and its high heat capacity. Additionally, water's polar nature allows it to surround and dissolve ions, facilitating various chemical reactions. Overall, electrostatic forces play a crucial role in shaping water's unique properties and its importance in biological and chemical systems.
Loading comments...
-
 LIVE
LIVE
Russell Brand
1 hour ago5 Years Late: NYT Admits We Were Lied to About COVID! – SF556
27,000 watching -
 LIVE
LIVE
Sean Unpaved
59 minutes agoOpening Day In The MLB! NFL Bans New Celebration Gesture, NCAA Sweet 16 Predictions!
517 watching -
 LIVE
LIVE
Nerdrotic
3 hours agoThe M-She-U is OVER and Marvel DUMPS Women - Nerdrotic Nooner 476
1,667 watching -
 LIVE
LIVE
Ben Shapiro
46 minutes agoEp. 2167 - MORE TRUMP WINNING: NPR CEO IMPLODES, MS-13 Head ARRESTED
1,893 watching -
 1:01:08
1:01:08
Timcast
1 hour agoMasked ICE "DISAPPEAR" Anti Israel Protester Migrant, Democrats FURIOUS Over Ice Deportations
60.1K108 -
 2:05:00
2:05:00
Steven Crowder
4 hours agoWhat Trump’s Massive Auto Tariffs Are Really About | Guest: Bill Richmond
255K235 -
 2:25:42
2:25:42
Right Side Broadcasting Network
4 hours agoLIVE REPLAY: Congress Holds Hearing: “INL Should Fight Crime, Not Fight Conservatives” - 3/27/25
17.5K11 -
 LIVE
LIVE
IrishBreakdown
1 hour agoNotre Dame Offensive Line Remains A Question Mark Through Spring
1,562 watching -
 LIVE
LIVE
TheAlecLaceShow
2 hours agoGuests: House Majority Leader Steve Scalise & Rep. Michael Guest | Signalgate | The Alec Lace Show
123 watching -
 1:47:22
1:47:22
Tate Speech by Andrew Tate
6 hours agoEMERGENCY MEETING EPISODE 107 - YOU’RE A NOBODY!
93.8K49