Premium Only Content
Prof. Nektarios Tavernarakis @ FLOGEN SIPS 2022 Yoshikawa Intl. Symposium on Oxidative Stress
FLOGEN SIPS 2022: Yoshikawa International Symposium on Oxidative Stress for Sustainable Development of Human Beings (2nd international Symposium)
Presenter:
Prof. Nektarios Tavernarakis, University of Crete, Heraklion, Greece
Title:
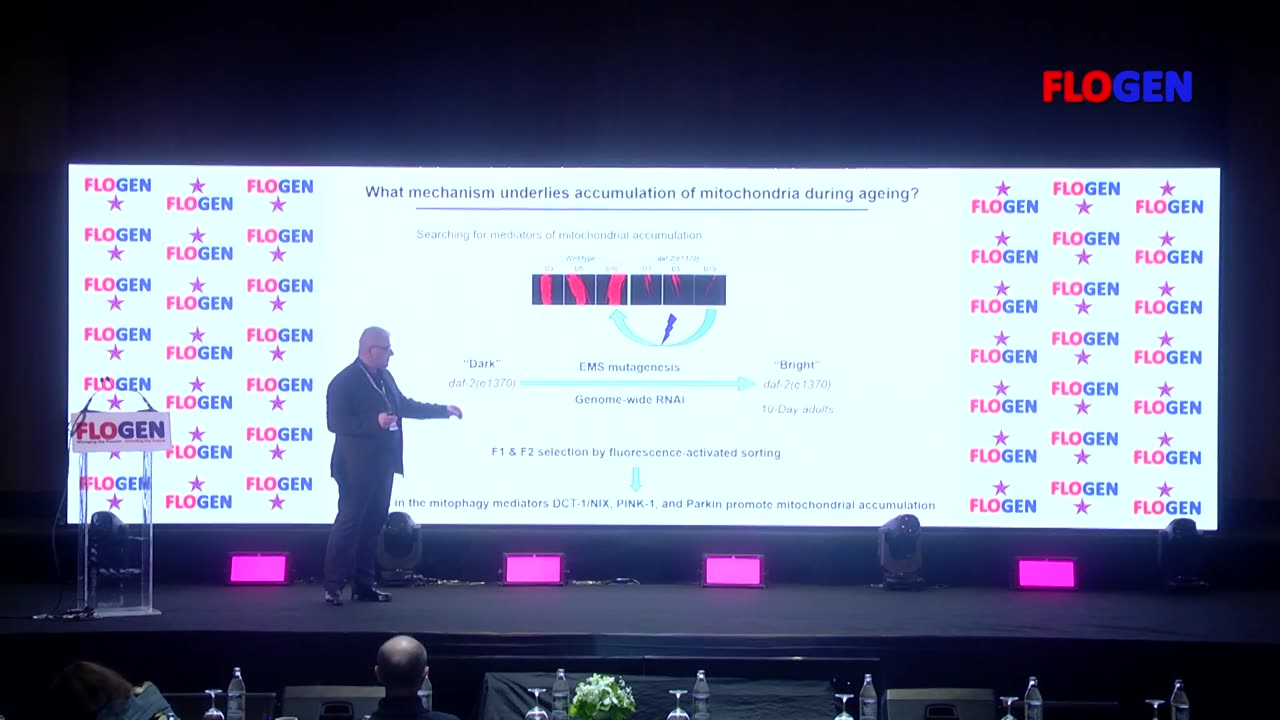
Cellular energy homeostasis in neurodegeneration and ageing
Abstract
Ageing is driven by the inexorable and stochastic accumulation of damage in biomolecules vital for proper cellular function. Although this process is fundamentally haphazard and uncontrollable, senescent decline and ageing is broadly influenced by genetic and extrinsic factors. Numerous gene mutations and treatments have been shown to extend the lifespan of diverse organisms ranging from the unicellular Saccharomyces cerevisiae to primates. It is becoming increasingly apparent that most such interventions ultimately interface with cellular stress response mechanisms, suggesting that longevity is intimately related to the ability of the organism to effectively cope with both intrinsic and extrinsic stress. Key determinants of this capacity are the molecular mechanisms that link ageing to main stress response pathways, and mediate age-related changes in the effectiveness of the response to stress. How each pathway contributes to modulate the ageing process is not fully elucidated. A better understanding of the dynamics and reciprocal interplay between stress responses and ageing is critical for the development of novel therapeutic strategies that exploit endogenous stress combat pathways against age-associated pathologies. Mitochondria, the indispensable and highly dynamic, energy-generating organelles in all eukaryotic cells, play essential roles in fundamental cellular processes. Neuronal cells depend, perhaps more than any other cell type, on proper mitochondrial function. Mitochondrial impairment is a major hallmark of several age-related neurodegenerative pathologies, including Alzheimer’s disease. Interestingly, accumulation of damaged mitochondria has been observed in post-mortem brain of Alzheimer’s disease patients. Mitophagy is a selective type of autophagy mediating elimination of damaged mitochondria, and the major degradation pathway, by which cells regulate mitochondrial number in response to their metabolic state. However, little is known about the role of mitophagy in the pathogenesis of Alzheimer’s disease. Although disease-associated tau and amyloid β are known to deregulate mitochondrial function, it remains elusive whether they also directly influence the efficiency of mitophagy. To address this question, we developed an in vivo imaging system to monitor mitophagy in neurons. We demonstrated that neuronal mitophagy is impaired in C. elegans models of Alzheimer’s disease. Urolithin A- and nicotinamide mononucleotide-induced mitophagy ameliorates several pathological features of Alzheimer’s disease, including cognitive defects. Mitophagy stimulation restores memory impairment. Age-dependent decline of mitophagy both inhibits removal of dysfunctional or superfluous mitochondria and impairs mitochondrial biogenesis resulting in progressive mitochondrial accretion and consequently, deterioration of cell function. Our findings suggest that impaired removal of damaged mitochondria is a pivotal event in Alzheimer’s disease pathogenesis highlighting mitophagy as a potential therapeutic intervention.
-
 12:29
12:29
Mr. Build It
5 days agoWish I Knew This Before I Started Building It
31.3K18 -
 2:03:57
2:03:57
Megyn Kelly
2 days agoNew Trump Derangement Syndrome, and How CNN Smeared a Navy Veteran, w/ Piers Morgan & Zachary Young
127K170 -
 10:05
10:05
DIY Wife
3 years agoHow We Flip Old Furniture For Profit!
65.3K58 -
 2:14:54
2:14:54
TheSaltyCracker
8 hours agoTrump Goes Gangster ReeEEeE Stream 01-26-25
139K296 -
 4:42:13
4:42:13
Due Dissidence
18 hours agoTrump Calls To "CLEAN OUT" Gaza, Swiss ARREST Pro-Palestine Journalist, MAGA's Hollywood Makeover?
63.1K85 -
 2:02:20
2:02:20
Nerdrotic
10 hours ago $19.44 earnedDECLASSIFIED: JFK, MLK UFO Immaculate Constellation Doc | Forbidden Frontier #089
84.8K17 -
 3:00:14
3:00:14
vivafrei
18 hours agoEp. 248: "Bitcoin Jesus" Begs Trump! Rekieta Gets Plea Deal! Pardons, Deportations, Bird Flu & MORE!
197K214 -
 3:44:06
3:44:06
Rising Rhino
17 hours ago $13.83 earnedWashington Commanders Vs Philadelphia Eagles: NFL NFC Championship LIVE Watch Party
93.3K4 -
 13:00
13:00
Exploring With Nug
11 hours ago $7.04 earnedHe Went To Get A Haircut And Vanished WIthout a Trace!
75.5K3 -
 18:53
18:53
DeVory Darkins
2 days ago $33.42 earnedTrump JUST ENDED Mayor Karen Bass During HEATED Meeting
104K223