Premium Only Content
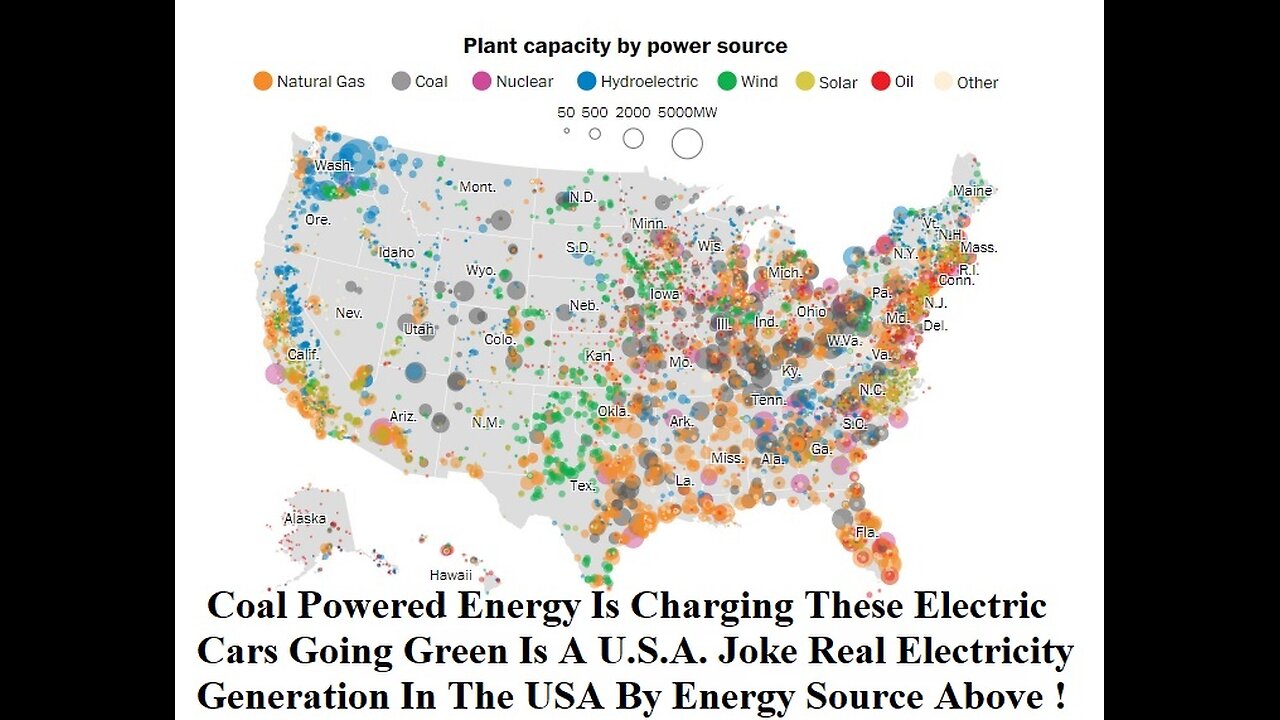
Going Green Is A U.S.A. Joke Real Electricity Generation In The US By Energy Source
Going Green Is A U.S.A. Joke ? So Is Coal Powered Car The Real Future Of An Electric Car Today ? or is electric vehicle (EV) is a passenger automobile that is propelled by an electric traction motor, using only energy stored in on-board batteries. Compared to conventional internal combustion engine (ICE) vehicles, electric cars are quieter, more responsive, have superior energy conversion efficiency and no exhaust emissions and lower overall vehicle emissions (however the power plant supplying the electricity might generate its own emissions.
Coal is partially used to power electric vehicles, along with nuclear, solar, and wind energy. Electric cars are charged up by connecting them to the electric grid through any outlet in your home. Depending on the country, the larger electrical grid is powered by a variety of different sources. While electric vehicles themselves produce zero carbon emissions, charging them increases the amount of electricity generated from fossil fuel energy sources such as coal. A joint study conducted by researchers at the universities of Cambridge, Exeter, and Nijmegen concluded that driving an electric vehicle is better for the environment than driving a gas-powered car in 95% of the world. However, this does not include areas like Poland, where electricity is mostly based on coal. Disdain for electric cars runs deep in some states.
The electricity sector of the United States includes a large array of stakeholders that provide services through electricity generation, transmission, distribution, and marketing for industrial, commercial, public, and residential customers. It also includes many public institutions that regulate the sector. In 1996, there were 3,195 electric utilities in the United States, of which fewer than 1,000 were engaged in power generation. This leaves a large number of mostly smaller utilities engaged only in power distribution. There were also 65 power marketers. Of all utilities, 2,020 were publicly owned (including 10 Federal utilities), 932 were rural electric cooperatives, and 243 were investor-owned utilities. The electricity transmission network is controlled by Independent System Operators or Regional Transmission Organizations, which are not-for-profit organizations that are obliged to provide indiscriminate access to various suppliers in order to promote competition.
Principal sources of US electricity in 2019 were: natural gas (38%), coal (23%), nuclear (20%), other renewables (11%), and hydro (7%). Over the decade 2004–2014, the largest increases in electrical generation came from natural gas (2014 generation was 412 TWh greater than 2004), wind (an increase of 168 TWh), and solar (increased 18 TWh). Over the same decade, annual generation from coal decreased 393 TWh, and from petroleum decreased 90 TWh.
https://docs.wind-watch.org/Benton-PUD-Policy-Perspectives-2020-July-14.pdf
10 MYTHS ABOUT CLIMATE CHANGE
With the climate crisis becoming a hot topic in mainstream media - there's a lot of confusion around what climate change actually is. That's why we've tried to clear up some of the most frequently heard myths, so that you can tell fiction from fact!
MYTH 1. THE EARTH’S CLIMATE HAS ALWAYS CHANGED
Over the course of the Earth’s 4.5-billion-year history, the climate has changed a lot. This is true. But the rapid warming we’re seeing now can't be explained by natural cycles of warming and cooling. The kind of changes that would normally happen over hundreds of thousands of years are happening in decades.
Global temperatures are now at their highest since records began. In fact, 17 of the 18 warmest years on record have all taken place since 2001.
This much faster warming corresponds with levels of carbon dioxide in the atmosphere, which have been increasing since the industrial revolution. So, when people talk about climate change today, they mean anthropogenic (man-made) climate change. This is the warming of Earth’s average temperature as a result of human activity, such as burning coal, oil and gas to produce energy to fuel our homes and transport and cutting down trees to produce the food we eat.
MYTH 2. PLANTS NEED CARBON DIOXIDE
Plants do need carbon dioxide (CO2) to live. Plants and forests remove and store away huge amounts of carbon dioxide from the atmosphere each year. But the problem is, there’s only so much carbon dioxide they can absorb and this amount is getting less, as more and more forests are cut down across the world, largely to produce our food.
Let’s be clear, CO2 itself does not cause problems. It's part of the natural global ecosystem. The problem is the quantity of CO2 that’s being produced by us as humans; there hasn’t been this level of CO2 in the atmosphere for 800,000 years.
MYTH 3. GLOBAL WARMING ISN'T REAL AS IT'S STILL COLD
Global warming is causing the Earth’s average surface temperature to increase. This is not only making heatwaves and droughts more likely but it’s also causing changes to our natural climate systems. These changes are making extreme weather events more likely and more severe. For example, hurricanes and storms are becoming more intense, moving slower and taking longer to die down.
Because of where we are, the UK & Ireland are likely to get more rain and wind as a result of climate change while New York will see more snow.
MYTH 4. CLIMATE CHANGE IS A FUTURE PROBLEM
This is no longer an excuse not to act on climate change and push the burden onto future generations. Last year, the world’s leading climate scientists warned we only have 12 years to limit global warming to a maximum of 1.5C and avoid climate breakdown.
We’re already seeing the devastating effects of climate change on global food supplies, increasing migration, conflict, disease and global instability, and this will only get worse if we don’t act now. Man-made climate change is the biggest environmental crisis of our time. It threatens the future of the planet that we depend on for our survival and we're the last generation that can do something about it.
MYTH 5. RENEWABLE ENERGY IS JUST A MONEY-MAKING SCHEME
It's a commonly-held belief that renewable energy is expensive, but this simply isn’t true! Solar power and onshore wind are the cheapest ways of generating electricity; meaning the energy they produce is cheaper than using nuclear, gas and fossil fuels.
The cost of renewables has fallen faster than anyone could have predicted. And yet the government are still backing dirty fossil fuels. Did you know the UK has the biggest fossil fuel subsidies in the EU? That’s right, they spend an eye-watering €12bn (£10.5bn) a year supporting dirty fossil fuels.
MYTH 6. POLAR BEAR NUMBERS ARE INCREASING
This isn’t the case. Climate change is the biggest threat faced by polar bears. The Arctic is warming roughly four times faster than the rest of the world, causing sea ice to melt earlier and form later each year. This makes it more difficult for female polar bears to get onto land in late autumn to build their dens, and more difficult for them to get out onto the sea ice in spring to feed their cubs. Their main source of prey, seals, are also affected by climate change, as they depend on sea ice to raise their young.
This means that in some parts of the Arctic, polar bears are having to survive with less food than they did previously. Polar bear populations are predicted to decline by 30% by the middle of this century.
MYTH 7. RENEWABLE ENERGY CAN ONLY WORK WHEN IT'S NOT CLOUDY OR WINDY
Industry is developing new techniques for storing electricity and managing demand at peak times meaning that even if the sun isn't shining or it’s not blowing a gale, it’s still possible to rely on renewable energy sources.
The majority of UK homes get their electricity from the National Grid. When you switch to a clean supplier, they guarantee that for every unit of electricity you take out of the Grid, they’ll put the same amount of clean energy back in, helping to clean up our energy supply.
MYTH 8. ANIMALS WILL ADAPT TO CLIMATE CHANGE
This one isn't a myth, Darwin got the adaptation part right. But let’s be clear, some plants and animals will adapt but not all.
To survive, plants, animals and birds confronted with climate change have two options: move or adapt. There are several examples of species that have begun to adapt to climate change already.
But increasingly, it's a different story for many. Given the speed of climate change, it’s becoming impossible for many species to adapt quickly enough to keep up with their changing environment. And, as habitats are destroyed by roads, cities and dams, moving becomes increasingly difficult. For those that can’t move or adapt, the future doesn’t look so positive.
MYTH 9. GETTING RID OF HUMANS WILL FIX THIS
This, we firmly believe, is wrong. It’s easy to start feeling that we've gone too far already and that the planet won’t be able to support the world’s growing population.
It's WWF's mission to build a world where people and nature thrive together. The technology and systems we need to move to 100% renewable energy by 2045 and use our planet's resources sustainably are already available. What's now needed is for political and business leaders to take bold and urgent action towards using these solutions to address the climate crisis and restore nature.
MYTH 10. CHINA IS THE ONLY COUNTRY RESPONSIBLE FOR CLIMATE CHANGE
Despite being one of the largest emitters of greenhouse gases, China is currently one of the largest investors in renewables. The increase in investment has been in response to the rapid growth of green business and the need to clean up air pollution in its major cities.
Climate change is a global issue and we all have a responsibility to step up to the climate crisis. Action on it will need serious investment but has the potential to deliver huge benefits for nature and people. We all need to raise our voices and fight for our world!
Did you know that the food we eat has a massive impact on the health of our planet?
Food production is responsible for 37% of all greenhouse gas emissions, which are causing our home to warm far too quickly. This means habitats are at risk, sea levels are rising, more extreme weather is causing floods and droughts, and our lives as we currently know them are under threat. If we want a healthy planet, we all need to be smarter about what we eat and how it’s produced.
Mapping how the United States generates its electricity signed orders to reverse the previous administration’s energy policies, a move that he framed as “an end to the war on coal” and that comes amid a drop in the fuel’s use. Natural gas surpassed coal last year as the most common source for electricity generation in the United States, according to a Post analysis of preliminary data from the Energy Information Administration. Coal was responsible for a majority of electricity generation at the start of the century and was still the source for nearly half in 2008 but has fallen steadily, accounting for 30 percent last year. Natural gas powered 34 percent of the country's electricity last year, passing coal as well as nuclear.
Local electric utilities take advantage of nearby resources — rivers in the Northwest, wind in the Midwest, coal in the Appalachian region, natural gas in the North — to generate the bulk of the nation’s electricity. This shows the source of electricity generation in each state according to preliminary data.
Natural gas-powered electric plants There are 1,793 natural gas-powered electricity plants in the United States. They generated 34 percent of the nation's electricity last year.
Advances and expansion of fracking in the past decade have unlocked vast supplies of natural gas from shale deposits all over the country. The fuel is the primary source of electricity generation in 19 states and provides at least 50 percent of the electricity in nine states.
There are 400 coal-powered electric plants in the United States. They generated 30 percent of the nation's electricity last year.
Coal was the chief source of electrical generation in 19 states and the second most common source in another nine. Coal is most popular in the East, south of New York. Coal still accounted for at least 50 percent of generation in 13 states.
There are 61 nuclear electric plants in the United States. They generated 20 percent of the nation’s electricity last year.
New nuclear plants are coming online following decades of pause after an initial push in the 1970s and 1980s driven by the first oil shock. Maryland joined South Carolina, Illinois, Pennsylvania, Connecticut and New Hampshire in getting a plurality of its power from nuclear last year. Twenty states have no nuclear electricity generation at all.
There are 1,444 hydroelectric plants in the United States. They generated 7 percent of the nation’s electricity last year.
It’s a feast-or-famine source. Washington, Oregon, Vermont and Idaho lead the nation in power from hydroelectric plants, getting between 56 percent and 68 percent of their electricity from them. But Montana and South Dakota were the only other states where they were responsible for more than 5 percent of electricity. Government-run plants generate most of the power.
There are 999 wind-powered electric plants in the United States. They generated 6 percent of the nation’s electricity last year.
Wind is the fastest-growing power source, finding a home in the Great Plains, where wind blows reliably across wide open spaces. Iowa got more than one-third of its power from wind, followed by Kansas, Oklahoma and South Dakota, which each got more than a quarter of their electricity from windmills. Wind is not the leading source of electric power anywhere but ranks second in seven states.
There are 1,721 solar-powered electric plants in the United States. They generated 1 percent of the nation’s electricity last year.
Solar power is predominantly used in the Southwest, where the sun shines the most. The growth of solar has created plants in all but eight states. California gets almost 10 percent of its electricity from solar, and Nevada gets more than 6 percent. Vermont and Arizona follow with 4 percent each.
There are 1,076 oil-powered electric plants in the United States. They generated just over half of 1 percent of the nation’s electricity last year.
Petroleum is no longer a popular source for electricity generation. After the rise of OPEC and the oil shocks and price increases of the 1970s, utilities switched to other fuels, mostly coal. Hawaii gets two-thirds of its electricity from oil, the only state where it is the leading energy source.
Coal-Powered Energy Charging These Electric Cars One of the knocks against battery-electric vehicles is that they typically recharge from a fossil-fuel-powered grid, and therefore are no better for the environment than fossil-fuel-burning cars. And if that grid is powered mostly by coal – the dirtiest of the fossil fuels – then EVs might even be worse than conventional cars. Makes sense, right?
On the surface, sure. But what seems like airtight logic falls apart under scrutiny – because of EVs’ incredible efficiency and because of coal’s fading role in the generation of electricity.
Coal is rolling… on out of style
There are fewer and fewer U.S. grids left that rely primarily on coal-fired power. Whereas more than half of states used mostly coal for electricity in 2001, only 15 still do. According to the U.S. Energy Information Administration, nearly a quarter of remaining U.S. coal-fired generating operations have announced plans to shut down.
Coal has been on a long, steady decline as a percentage of all grid fuels in the U.S., falling from about 45% in 2010 to 21% in 2022. During the same period, renewables have doubled, rising from 10% to 20%. Natural gas remains the largest single fuel source for the U.S. grid, and while its share of the mix has expanded in the last 12 years, its growth rate trails that of renewables. Political forces will surely continue to intervene, but the low cost of renewables all but assures the growth of wind, solar and other low-carbon sources at the expense of traditional fuels.
Making an EV is the dirtiest part
Most studies agree that manufacturing an EV is a more carbon-intensive process than building a vehicle with an internal combustion engine. But once the cars are assembled and delivered to the customer, the ICE’s environmental advantage begins to unravel. Between 8,400 and 13,500 miles of driving, the ICE vehicle’s carbon footprint overtakes that of the EV and continues to grow ever larger, while the future carbon emissions from the EV are marginal and depend on your local electric grid. The EV extends its advantage even when it is repeatedly charging from a grid powered mostly by fossil fuels, including coal.
What this means is that once an EV has been on the road a year or two, it has “broken even” with an ICE car in terms of emissions. The ICE continues to add to its lifetime carbon emissions, while the EV barely does.
It all comes down to efficiency
How is it possible that an EV is cleaner regardless of how its energy is produced? The answer lies in EVs’ profound efficiency advantages over ICE vehicles. No matter the trip, whether you’re puttering around town or rocketing down the Interstate, the EV consumes fuel at a radically lower rate. The slower consumption generates far fewer emissions for each mile driven.
The efficiency differential between EVs and comparable conventional vehicles is extreme.
An example: The compact Honda Civic, long celebrated as a champion of thrift, averages 36 MPG, according to the EPA. The Tesla Model 3, an electric sedan similar in size to the Civic, delivers the equivalent of 132 MPG (expressed by the EPA as MPG-equivalent, or MPGe).
That's a whopping 367% efficiency advantage over the miserly Honda.
In case you’re thinking the Tesla might be an outlier, there are 17 EV models available in the U.S. that deliver economy above 100 MPGe, with six achieving 120 MPGe or better and two exceeding 130. No gas-powered vehicle comes close. The Hyundai Ionic Blue hybrid, the current efficiency champion among fossil-fueled vehicles, rings in at 59 MPG. This means that while a gallon of gas gets an Ioniq Blue 59 miles, the same amount of energy gets most EVs 100 miles.
If you’re really trying to drive carbon neutral, a used EV will be the cleanest option, since no new production is required. So, if you’re shopping for something, say, around two years old, you’ll be happy to learn that the 10 best-selling EVs of 2020 averaged the equivalent of 108 miles per gallon. Read more about highly efficient EVs or the way that driving style may influence efficiency.
EVs are cleaner now, and getting better
A study by the International Council on Clean Transportation found that “battery electric vehicles (BEVs) have by far the lowest life-cycle [greenhouse gas] emissions. Emissions over the lifetime of average medium-size BEVs registered today are already lower than comparable gasoline cars by 66%–69% in Europe, 60%–68% in the United States, 37%–45% in China, and 19%–34% in India.” The difference between emissions savings in different regions comes down to how dirty it is to produce energy in each of those places.
The ICCT study concludes that “…as the electricity mix continues to decarbonize, the life-cycle emissions gap between BEVs and gasoline vehicles increases substantially,” and that EVs entirely powered by renewable energy would correspond to 81% lower lifecycle GHG emissions than gasoline cars. Coal-powered EVs? The point is nearly moot.
Overview of Greenhouse Gases Gases that trap heat in the atmosphere are called greenhouse gases. This section provides information on emissions and removals of the main greenhouse gases to and from the atmosphere.
Carbon dioxide (CO2): Carbon dioxide enters the atmosphere through burning fossil fuels (coal, natural gas, and oil), solid waste, trees and other biological materials, and also as a result of certain chemical reactions (e.g., cement production). Carbon dioxide is removed from the atmosphere (or "sequestered") when it is absorbed by plants as part of the biological carbon cycle.
Methane (CH4): Methane is emitted during the production and transport of coal, natural gas, and oil. Methane emissions also result from livestock and other agricultural practices, land use, and by the decay of organic waste in municipal solid waste landfills.
Nitrous oxide (N2O): Nitrous oxide is emitted during agricultural, land use, and industrial activities; combustion of fossil fuels and solid waste; as well as during treatment of wastewater.
Fluorinated gases: Hydrofluorocarbons, perfluorocarbons, sulfur hexafluoride, and nitrogen trifluoride are synthetic, powerful greenhouse gases that are emitted from a variety of household, commercial, and industrial applications and processes. Fluorinated gases (especially hydrofluorocarbons) are sometimes used as substitutes for stratospheric ozone-depleting substances (e.g., chlorofluorocarbons, hydrochlorofluorocarbons, and halons). Fluorinated gases are typically emitted in smaller quantities than other greenhouse gases, but they are potent greenhouse gases. With global warming potentials (GWPs) that typically range from thousands to tens of thousands, they are sometimes referred to as high-GWP gases because, for a given amount of mass, they trap substantially more heat than CO2.
Each gas's effect on climate change depends on three main factors:
How abundant are greenhouse gases in the atmosphere?
Concentration, or abundance, is the amount of a particular gas in the air. Larger emissions of greenhouse gases lead to higher concentrations in the atmosphere. Greenhouse gas concentrations are measured in parts per million, parts per billion, and even parts per trillion. One part per million is equivalent to one drop of water diluted into about 13 gallons of liquid (roughly the fuel tank of a compact car). To learn more about the increasing concentrations of greenhouse gases in the atmosphere, visit the Climate Change Indicators: Atmospheric Concentrations of Greenhouse Gases page.
How long do greenhouse gases stay in the atmosphere?
Each of these gases can remain in the atmosphere for different amounts of time, ranging from a few years to thousands of years. All of these gases remain in the atmosphere long enough to become well mixed, meaning that the amount that is measured in the atmosphere is roughly the same all over the world, regardless of the source of the emissions.
How strongly do greenhouse gases impact the atmosphere?
Some gases are more effective than others at making the planet warmer and "thickening the Earth's atmospheric blanket."
For each greenhouse gas, a Global Warming Potential (GWP) was developed to allow comparisons of the global warming impacts of different gases. Specifically, it is a measure of how much energy the emissions of 1 ton of a gas will absorb over a given period of time, typically a 100-year time horizon, relative to the emissions of 1 ton of carbon dioxide (CO2). Gases with a higher GWP absorb more energy, per ton emitted, than gases with a lower GWP, and thus contribute more to warming Earth.
Note: All emission estimates are from the Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2021. The Inventory uses 100-year GWPs from IPCC’s Fifth Assessment Report (AR5).
Carbon Dioxide Emissions
Carbon dioxide (CO2) is the primary greenhouse gas emitted through human activities. In 2021, CO2 accounted for 79% of all U.S. greenhouse gas emissions from human activities. Carbon dioxide is naturally present in the atmosphere as part of the Earth's carbon cycle (the natural circulation of carbon among the atmosphere, oceans, soil, plants, and animals). Human activities are altering the carbon cycle–both by adding more CO2 to the atmosphere and by influencing the ability of natural sinks, like forests and soils, to remove and store CO2 from the atmosphere. While CO2 emissions come from a variety of natural sources, human-related emissions are responsible for the increase that has occurred in the atmosphere since the industrial revolution.
The main human activity that emits CO2 is the combustion of fossil fuels (coal, natural gas, and oil) for energy and transportation. Certain industrial processes and land-use changes also emit CO2. The main sources of CO2 emissions in the United States are described below.
Transportation. The combustion of fossil fuels such as gasoline and diesel to transport people and goods was the largest source of CO2 emissions in 2021, accounting for 35% of total U.S. CO2 emissions and 28% of total U.S. greenhouse gas emissions. This category includes domestic transportation sources such as highway and passenger vehicles, air travel, marine transportation, and rail.
Electricity. Electricity is a key source of energy in the United States and is used to power homes, business, and industry. In 2021, the combustion of fossil fuels to generate electricity was the second largest source of CO2 emissions in the nation, accounting for 31% of total U.S. CO2 emissions and 24% of total U.S. greenhouse gas emissions. The types of fossil fuel used to generate electricity emit different amounts of CO2. To produce a given amount of electricity, burning coal will produce more CO2 than natural gas or oil.
Industry. Many industrial processes emit CO2 through fossil fuel consumption. Several processes also produce CO2 emissions through chemical reactions that do not involve combustion, and examples include the production of mineral products such as cement, the production of metals such as iron and steel, and the production of chemicals. The fossil fuel combustion component of various industrial processes accounted for 15% of total U.S. CO2 emissions and 12% of total U.S. greenhouse gas emissions in 2021. Many industrial processes also use electricity and therefore indirectly result in CO2 emissions from electricity generation.
Carbon dioxide is constantly being exchanged among the atmosphere, ocean, and land surface as it is both produced and absorbed by many microorganisms, plants, and animals. Emissions and removals of CO2 by these natural processes, however, tend to balance over time, absent anthropogenic impacts. Since the Industrial Revolution began around 1750, human activities have contributed substantially to climate change by adding CO2 and other heat-trapping gases to the atmosphere.
In the United States, the management of forests and other land (e.g., cropland, grasslands, etc.) has acted as a net sink of CO2, which means that more CO2 is removed from the atmosphere, and stored in plants and trees, than is emitted. This carbon sink offset about 13% of total emissions in 2021. For more details, see the discussion in the Land Use, Land-Use Change, and Forestry section.
To find out more about the role of CO2 in warming the atmosphere and its sources, visit the Climate Change Indicators page.
Trends
Carbon dioxide emissions in the United States decreased by 2% between 1990 and 2021. Since the combustion of fossil fuel is the largest source of greenhouse gas emissions in the United States, changes in emissions from fossil fuel combustion have historically been the dominant factor affecting total U.S. emission trends. Changes in CO2 emissions from fossil fuel combustion are influenced by many long-term and short-term factors, including population growth, economic growth, changing energy prices, new technologies, changing behavior, and seasonal temperatures. In 2021, the increase in CO2 emissions from fossil fuel combustion corresponded with an increase in energy use as a result of economic activity rebounding after the height of the COVID-19 pandemic, in addition to an increase in coal use in the electric power sector.
Reducing Carbon Dioxide Emissions
The most effective way to reduce CO2 emissions is to reduce fossil fuel consumption. Many strategies for reducing CO2 emissions from energy are cross-cutting and apply to homes, businesses, industry, and transportation.
Atmospheric CO2 is part of the global carbon cycle, and therefore its fate is a complex function of geochemical and biological processes. Some of the excess carbon dioxide will be absorbed quickly (for example, by the ocean surface), but some will remain in the atmosphere for thousands of years, due in part to the very slow process by which carbon is transferred to ocean sediments.
Methane Emissions
In 2021, methane (CH4) accounted for 12% of all U.S. greenhouse gas emissions from human activities. Human activities emitting methane include leaks from natural gas systems and the raising of livestock. Methane is also emitted by natural sources such as termites. In addition, natural processes in soil and chemical reactions in the atmosphere help remove CH4 from the atmosphere. Methane's lifetime in the atmosphere is much shorter than carbon dioxide (CO2), but CH4 is more efficient at trapping radiation than CO2. Pound for pound, the comparative impact of CH4 is 28 times greater than CO2 over a 100-year period.1
Globally, 50-65% of total CH4 emissions come from human activities.2 Methane is emitted from energy, industry, agriculture, land use, and waste management activities, described below.
Agriculture. Domestic livestock such as cattle, swine, sheep, and goats produce CH4 as part of their normal digestive process. Also, when animal manure is stored or managed in lagoons or holding tanks, CH4 is produced. Because humans raise these animals for food and other products, the emissions are considered human-related. The Agriculture sector is the largest source of CH4 emissions in the United States.
LULUCF: While not shown in the figure, emissions of CH4 also occur as a result of land use and land management activities in the Land Use, Land-Use Change, and Forestry sector (e.g. forest and grassland fires, management of flooded lands such as reservoirs, decomposition of organic matter in coastal wetlands).
Energy and Industry. Natural gas and petroleum systems are the second largest source of CH4 emissions in the United States. Methane is emitted to the atmosphere during the production, processing, storage, transmission, distribution, and use of natural gas, and the production, refinement, transportation, and storage of crude oil. Coal mining is also a source of CH4 emissions. For more information, see the Inventory of U.S. Greenhouse Gas Emissions and Sinks sections on Natural Gas Systems and Petroleum Systems.
Waste from Homes and Businesses. Methane is generated in landfills as waste decomposes and in the treatment of wastewater. Landfills are the third-largest source of CH4 emissions in the United States. Methane is also generated from domestic and industrial wastewater treatment and from composting and anaerobic digestion. For more information, see the Inventory of U.S. Greenhouse Gas Emissions and Sinks Waste chapter.
Methane is also emitted from a number of natural sources. Natural wetlands that are not managed or changed by human activity are the largest source, emitting CH4 from bacteria that decompose organic materials in the absence of oxygen. Reservoirs and ponds with high organic matter and low oxygen levels also produce methane through the microbial breakdown of organic matter. Smaller sources include termites, oceans, sediments, volcanoes, and wildfires.
Methane emissions in the United States decreased by 16% between 1990 and 2021. During this time period, emissions increased from sources associated with agricultural activities, while emissions decreased from other sources including landfills and coal mining and from natural gas and petroleum systems.
Reducing Methane Emissions
There are a number of ways to reduce CH4 emissions. Some examples are discussed below. EPA has a series of voluntary programs for reducing CH4 emissions, in addition to regulatory initiatives. EPA also supports the Global Methane Initiative, an international partnership encouraging global methane reduction strategies.
Nitrous Oxide Emissions
In 2021, nitrous oxide (N2O) accounted for 6% of all U.S. greenhouse gas emissions from human activities. Human activities such as agriculture, fuel combustion, wastewater management, and industrial processes are increasing the amount of N2O in the atmosphere. Nitrous oxide is also naturally present in the atmosphere as part of the Earth's nitrogen cycle and has a variety of natural sources. Nitrous oxide molecules stay in the atmosphere for an average of 121 years before being removed by a sink or destroyed through chemical reactions. The impact of 1 pound of N2O on warming the atmosphere is 265 times that of 1 pound of carbon dioxide.1
Globally, 40% of total N2O emissions come from human activities.2 Nitrous oxide is emitted from agriculture, land use, transportation, industry, and other activities.
Agriculture. Nitrous oxide can result from various agricultural soil management activities, such as application of synthetic and organic fertilizers and other cropping practices, the management of manure, or burning of agricultural residues. Agricultural soil management is the largest source of N2O emissions in the United States, accounting for 75% of total U.S. N2O emissions in 2021. While not shown in the figure and less significant, emissions of N2O also occur as a result of land use and land management activities in the Land Use, Land-Use Change, and Forestry sector (e.g. forest and grassland fires, application of synthetic nitrogen fertilizers to urban soils (e.g., lawns, golf courses) and forest lands, etc.).
Fuel Combustion. Nitrous oxide is emitted when fuels are burned. The amount of N2O emitted from burning fuels depends on the type of fuel and combustion technology, maintenance, and operating practices.
Industry. Nitrous oxide is generated as a byproduct during the production of chemicals such as nitric acid, which is used to make synthetic commercial fertilizer, and in the production of adipic acid, which is used to make fibers, like nylon, and other synthetic products. Nitrous oxide is also emitted from use in other applications such as anesthesia and semiconductor manufacturing.
Waste. Nitrous oxide is also generated from treatment of domestic wastewater during nitrification and denitrification of the nitrogen present, usually in the form of urea, ammonia, and proteins.
Nitrous oxide emissions occur naturally through many sources associated with the nitrogen cycle, which is the natural circulation of nitrogen among the atmosphere, plants, animals, and microorganisms that live in soil and water. Nitrogen takes on a variety of chemical forms throughout the nitrogen cycle, including N2O. Natural emissions of N2O are mainly from bacteria breaking down nitrogen in soils and the oceans. Nitrous oxide is removed from the atmosphere when it is absorbed by certain types of bacteria or destroyed by ultraviolet radiation or chemical reactions.
Nitrous oxide emissions in the United States decreased by 3% between 1990 and 2021. During this time, nitrous oxide emissions from mobile combustion decreased by 56% as a result of criteria pollutant emission standards for on-road vehicles. Nitrous oxide emissions from agricultural soils have varied during this period and were about the same in 2021 as in 1990.
Unlike many other greenhouse gases, fluorinated gases have no significant natural sources and come almost entirely from human-related activities. They are emitted through their use as substitutes for ozone-depleting substances (e.g., as refrigerants) and through a variety of industrial processes such as aluminum and semiconductor manufacturing. Many fluorinated gases have very high global warming potentials (GWPs) relative to other greenhouse gases, so small atmospheric concentrations can nevertheless have large effects on global temperatures. They can also have long atmospheric lifetimes—in some cases, lasting thousands of years. Like other long-lived greenhouse gases, most fluorinated gases are well-mixed in the atmosphere, spreading around the world after they are emitted. Many fluorinated gases are removed from the atmosphere only when they are destroyed by sunlight in the upper atmosphere. In general, fluorinated gases are the most potent and longest lasting type of greenhouse gases emitted by human activities.
There are four main categories of fluorinated gases—hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), sulfur hexafluoride (SF6), and nitrogen trifluoride (NF3).
Substitution for Ozone-Depleting Substances. Hydrofluorocarbons are used as refrigerants, aerosol propellants, foam blowing agents, solvents, and fire retardants. The major emissions source of these compounds is their use as refrigerants—for example, in air conditioning systems in both vehicles and buildings. These chemicals were developed as a replacement for chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) because they do not deplete the stratospheric ozone layer. CFCs and HCFCs are also greenhouse gases; however, their contribution is not included here because they are being phased out under an international agreement called the Montreal Protocol. HFCs are potent greenhouse gases with high GWPs, and they are released into the atmosphere during manufacturing processes and through leaks, servicing, and disposal of equipment in which they are used. Newly developed hydrofluoroolefins (HFOs) are a subset of HFCs and are characterized by short atmospheric lifetimes and lower GWPs. HFOs are currently being introduced as refrigerants, aerosol propellants and foam blowing agents. The American Innovation and Manufacturing (AIM) Act of 2020 directs EPA to address HFCs by providing new authorities in three main areas: to phase down the production and consumption of listed HFCs in the United States by 85% over the next 15 years, manage these HFCs and their substitutes, and facilitate the transition to next-generation technologies that do not rely on HFCs.
Industry. Perfluorocarbons are produced as a byproduct of aluminum production and are used in the manufacturing of semiconductors. PFCs generally have long atmospheric lifetimes and GWPs near 10,000. Sulfur hexafluoride is used in magnesium processing and semiconductor manufacturing, as well as a tracer gas for leak detection. Nitrogen trifluoride is used in semiconductor manufacturing. HFC-23 is produced as a byproduct of HCFC-22 production and is used in semiconductor manufacturing.
Transmission and Distribution of Electricity. Sulfur hexafluoride is used as an insulating gas in electrical transmission equipment, including circuit breakers. The GWP of SF6 is 23,500 making it the most potent greenhouse gas that the Intergovernmental Panel on Climate Change has evaluated.
Overall, fluorinated gas emissions in the United States have increased by 105% between 1990 and 2021. This increase has been driven by a 349% increase in emissions of hydrofluorocarbons (HFCs) since 1990, as they have been widely used as a substitute for ozone-depleting substances. Emissions of perfluorocarbons (PFCs) and sulfur hexafluoride (SF6) have declined during this time due to emission-reduction efforts in the aluminum production industry (PFCs) and the electrical transmission and distribution industry (SF6)
6,340 million metric tons of CO2: What does that mean?
An explanation of units:
A million metric tons equals about 2.2 billion pounds, or 1 trillion grams. For comparison, a small car is likely to weigh a little more than 1 metric ton. Thus, a million metric tons is roughly the same mass as 1 million small cars!
The U.S. Inventory uses metric units for consistency and comparability with other countries. For reference, a metric ton is slightly more (approximately 10%) than a U.S. "short" ton.
GHG emissions are often measured in carbon dioxide (CO2) equivalent. To convert emissions of a gas into CO2 equivalent, its emissions are multiplied by the gas's Global Warming Potential (GWP). The GWP takes into account the fact that many gases are more effective at warming Earth than CO2, per unit mass.
How Exactly Does Carbon Dioxide Cause Global Warming?
So You Asked where Earth Institute experts tackle questions on science and sustainability. Over the past few years, we’ve received a lot of questions about carbon dioxide — how it traps heat, how it can have such a big effect if it only makes up a tiny percentage of the atmosphere, and more. With the help of Jason Smerdon, a climate scientist at Columbia University’s Lamont-Doherty Earth Observatory, we answer several of those questions here.
How does carbon dioxide trap heat?
You’ve probably already read that carbon dioxide and other greenhouse gases act like a blanket or a cap, trapping some of the heat that Earth might have otherwise radiated out into space. That’s the simple answer. But how exactly do certain molecules trap heat? The answer there requires diving into physics and chemistry.
simplified diagram of the greenhouse effect
Simplified diagram showing how Earth transforms sunlight into infrared energy. Greenhouse gases like carbon dioxide and methane absorb the infrared energy, re-emitting some of it back toward Earth and some of it out into space. Credit: A loose necktie on Wikimedia Commons
When sunlight reaches Earth, the surface absorbs some of the light’s energy and reradiates it as infrared waves, which we feel as heat. (Hold your hand over a dark rock on a warm sunny day and you can feel this phenomenon for yourself.) These infrared waves travel up into the atmosphere and will escape back into space if unimpeded.
Oxygen and nitrogen don’t interfere with infrared waves in the atmosphere. That’s because molecules are picky about the range of wavelengths that they interact with, Smerdon explained. For example, oxygen and nitrogen absorb energy that has tightly packed wavelengths of around 200 nanometers or less, whereas infrared energy travels at wider and lazier wavelengths of 700 to 1,000,000 nanometers. Those ranges don’t overlap, so to oxygen and nitrogen, it’s as if the infrared waves don’t even exist; they let the waves (and heat) pass freely through the atmosphere.
electromagnetic spectrum
A diagram showing the wavelengths of different types of energy. Energy from the Sun reaches Earth as mostly visible light. Earth reradiates that energy as infrared energy, which has a longer, slower wavelength. Whereas oxygen and nitrogen do not respond to infrared waves, greenhouse gases do. Credit: NASA
With CO2 and other greenhouse gases, it’s different. Carbon dioxide, for example, absorbs energy at a variety of wavelengths between 2,000 and 15,000 nanometers — a range that overlaps with that of infrared energy. As CO2 soaks up this infrared energy, it vibrates and re-emits the infrared energy back in all directions. About half of that energy goes out into space, and about half of it returns to Earth as heat, contributing to the ‘greenhouse effect.’
infrared radiation from different gases in the atmosphere
By measuring the wavelengths of infrared radiation that reaches the surface, scientists know that carbon dioxide, ozone, and methane are significantly contributing to rising global temperatures. Credit: Evans 2006 via Skeptical Science
Smerdon says that the reason why some molecules absorb infrared waves and some don’t “depends on their geometry and their composition.” He explained that oxygen and nitrogen molecules are simple — they’re each made up of only two atoms of the same element — which narrows their movements and the variety of wavelengths they can interact with. But greenhouse gases like CO2 and methane are made up of three or more atoms, which gives them a larger variety of ways to stretch and bend and twist. That means they can absorb a wider range of wavelengths — including infrared waves.
How can I see for myself that CO2 absorbs heat?
As an experiment that can be done in the home or the classroom, Smerdon recommends filling one soda bottle with CO2 (perhaps from a soda machine) and filling a second bottle with ambient air. “If you expose them both to a heat lamp, the CO2 bottle will warm up much more than the bottle with just ambient air,” he says. He recommends checking the bottle temperatures with a no-touch infrared thermometer. You’ll also want to make sure that you use the same style of bottle for each, and that both bottles receive the same amount of light from the lamp. Here’s a video of a similar experiment:
A more logistically challenging experiment that Smerdon recommends involves putting an infrared camera and a candle at opposite ends of a closed tube. When the tube is filled with ambient air, the camera picks up the infrared heat from the candle clearly. But once the tube is filled with carbon dioxide, the infrared image of the flame disappears, because the CO2 in the tube absorbs and scatters the heat from the candle in all directions, and therefore blurs out the image of the candle. There are several videos of the experiment online, including this one:
Why does carbon dioxide let heat in, but not out?
Energy enters our atmosphere as visible light, whereas it tries to leave as infrared energy. In other words, “energy coming into our planet from the Sun arrives as one currency, and it leaves in another,” said Smerdon.
CO2 molecules don’t really interact with sunlight’s wavelengths. Only after the Earth absorbs sunlight and reemits the energy as infrared waves can the CO2 and other greenhouse gases absorb the energy.
How can CO2 trap so much heat if it only makes up 0.04% of the atmosphere? Aren’t the molecules spaced too far apart?
Before humans began burning fossil fuels, naturally occurring greenhouse gases helped to make Earth’s climate habitable. Without them, the planet’s average temperature would be below freezing. So we know that even very low, natural levels of carbon dioxide and other greenhouse gases can make a huge difference in Earth’s climate.
Today, CO2 levels are higher than they have been in at least 3 million years. And although they still account for only 0.04% of the atmosphere, that still adds up to billions upon billions of tons of heat-trapping gas. For example, in 2019 alone, humans dumped 36.44 billion tonnes of CO2 into the atmosphere, where it will linger for hundreds of years. So there are plenty of CO2 molecules to provide a heat-trapping blanket across the entire atmosphere.
In addition, “trace amounts of a substance can have a large impact on a system,” explains Smerdon. Borrowing an analogy from Penn State meteorology professor David Titley, Smerdon said that “If someone my size drinks two beers, my blood alcohol content will be about 0.04 percent. That is right when the human body starts to feel the effects of alcohol.” Commercial drivers with a blood alcohol content of 0.04% can be convicted for driving under the influence.
“Similarly, it doesn’t take that much cyanide to poison a person,” adds Smerdon. “It has to do with how that specific substance interacts with the larger system and what it does to influence that system.”
In the case of greenhouse gases, the planet’s temperature is a balance between how much energy comes in versus how much energy goes out. Ultimately, any increase in the amount of heat-trapping means that the Earth’s surface gets hotter.
If there’s more water than CO2 in the atmosphere, how do we know that water isn’t to blame for climate change?
Water is indeed a greenhouse gas. It absorbs and re-emits infrared radiation, and thus makes the planet warmer. However, Smerdon says the amount of water vapor in the atmosphere is a consequence of warming rather than a driving force, because warmer air holds more water.
“We know this on a seasonal level,” he explains. “It’s generally drier in the winter when our local atmosphere is colder, and it’s more humid in the summer when it’s warmer.”
As carbon dioxide and other greenhouse gases heat up the planet, more water evaporates into the atmosphere, which in turn raises the temperature further. However, a hypothetical villain would not be able to exacerbate climate change by trying to pump more water vapor into the atmosphere, says Smerdon. “It would all rain out because temperature determines how much moisture can actually be held by the atmosphere.”
Similarly, it makes no sense to try to remove water vapor from the atmosphere, because natural, temperature-driven evaporation from plants and bodies of water would immediately replace it. To reduce water vapor in the atmosphere, we must lower global temperatures by reducing other greenhouse gases.
If Venus has an atmosphere that’s 95% CO2, shouldn’t it be a lot hotter than Earth?
Thick clouds of sulfuric acid surround Venus and prevent 75% of sunlight from reaching the planet’s surface. Without these clouds, Venus would be even hotter than it already is.
The concentration of CO2 in Venus’ atmosphere is about 2,400 times higher than that of Earth. Yet the average temperature of Venus is only about 15 times higher. What gives?
Interestingly enough, part of the answer has to do with water vapor. According to Smerdon, scientists think that long ago, Venus experienced a runaway greenhouse effect that boiled away almost all of the planet’s water — and water vapor, remember, is also a heat-trapping gas.
“It doesn’t have water vapor in its atmosphere, which is an important factor,” says Smerdon. “And then the other important factor is Venus has all these crazy sulfuric acid clouds.”
High up in Venus’ atmosphere, he explained, clouds of sulfuric acid block about 75% of incoming sunlight. That means the vast majority of sunlight never gets a chance to reach the planet’s surface, return to the atmosphere as infrared energy, and get trapped by all that CO2 in the atmosphere.
Won’t the plants, ocean, and soil just absorb all the excess CO2?
Eventually … in several thousand years or so.
Plants, the oceans, and soil are natural carbon sinks — they remove some carbon dioxide from the atmosphere and store it underground, underwater, or in roots and tree trunks. Without human activity, the vast amounts of carbon in coal, oil, and natural gas deposits would have remained stored underground and mostly separate from the rest of the carbon cycle. But by burning these fossil fuels, humans are adding a lot more carbon into the atmosphere and ocean, and the carbon sinks don’t work fast enough to clean up our mess.
It’s like watering your garden with a firehose. Even though plants absorb water, they can only do so at a set rate, and if you keep running the firehose, your yard is going to flood. Currently our atmosphere and ocean are flooded with CO2, and we can see that the carbon sinks can’t keep up because the concentrations of CO2 in the atmosphere and oceans are rising quickly.
chart showing rising co2 in the atmosphere
The amount of carbon dioxide in the atmosphere (raspberry line) has increased along with human emissions (blue line) since the start of the Industrial Revolution in 1750.
Unfortunately, we don’t have thousands of years to wait for nature to absorb the flood of CO2. By then, billions of people would have suffered and died from the impacts of climate change; there would be mass extinctions, and our beautiful planet would become unrecognizable. We can avoid much of that damage and suffering through a combination of decarbonizing our energy supply, pulling CO2 out the atmosphere, and developing more sustainable ways of thriving.
The World Is Warming All four major analyses of Earth’s average surface temperatures (NASA, NOAA, Hadcrut4, and Berkeley Earth) document global warming since 1950 of approximately 0.9 degrees Celsius (1.6 degrees Fahrenheit). There was very little change in temperature from 1950 to 1970. The world warmed 0.6 degrees from 1970 to 1998. There was very little change in temperature from 1998 through 2013. Then the world warmed an additional 0.3 degrees from 2013 to 2016, making 2016 the hottest year on record. Most climate scientists are convinced, based on greenhouse-warming theory, that this warming is caused primarily by increased burning of fossil fuels, causing increasing emissions of greenhouse gases, that are absorbing increasing amounts of infrared radiation emitted by Earth. Annual, average concentrations of carbon dioxide measured on Mauna Loa, Hawaii, have increased from 316 ppm in 1959 to 411 ppm in 2019 (30%). Most climate scientists are convinced that we must decrease burning of fossil fuels substantially and promptly in order to prevent dangerous overheating of Earth during the next few decades.
Why the Physics of Greenhouse-Warming Theory Appears to Be Mistaken
Greenhouse-warming theory, according to the Intergovernmental Panel on Climate Change, posits that greenhouse gases, primarily water vapor (H2O), carbon dioxide (CO2), nitrous oxide (N2O), methane (CH4) and ozone (O3)
“absorb terrestrial radiation emitted by Earth’s surface and elsewhere in the atmosphere. These substances emit infrared radiation in all directions, but, everything else being equal, the net amount emitted to space is normally less than would have been emitted in the absence of these absorbers. … An increase in the concentration of greenhouse gases increases the magnitude of this effect.”
Greenhouse-warming theory is thus based on the fundamental assumption described by Joseph Fourier in 1822 (Page 3) that the average temperature at Earth’s surface will increase if the net amount of terrestrial radiation reaching space becomes less than the net amount of solar radiation reaching Earth, often explained as the Earth-atmosphere energy balance, Earth’s energy budget, Earth’s radiation budget, tracking Earth’s energy, or Earth’s energy balance).
Since 1798, physicists have thought of heat as thermal energy in transfer—a flux through some surface measured in watts per square meter, where watts are the number of joules of energy passing through the surface each second. Climate scientists calculate radiative forcings, which are the net changes in flux (downward minus upward) caused by changes in concentrations in the atmosphere of each type of greenhouse gas, dust, black carbon soot, smoke from biomass burning, aerosols, volcanic aerosols, contrails, and such, or caused by any changes in radiation from Sun. They add these radiative forcings together to estimate changes in temperature.
Thus greenhouse-warming theory is based on the assumption that (1) radiative energy can be quantified by a single number of watts per square meter, (2) the assumption that these radiative forcings can be added together, and (3) the assumption that Earth’s surface temperature is proportional to the sum of all of these radiative forcings. A fundamentally new understanding of the physics of thermal energy and the physics of heat, described below, shows that all three assumptions are mistaken. There are other serious problems: (4) greenhouse gases absorb only a small part of the radiation emitted by Earth, (5) they can only reradiate what they absorb, (6) they do not reradiate in every direction as assumed, (7) they make up only a tiny part of the gases in the atmosphere, and (8) they have been shown by experiment not to cause significant warming. (9) The thermal effects of radiation are not about amount of radiation absorbed, as currently assumed, they are about the temperature of the emitting body and the difference in temperature between the emitting and the absorbing bodies as described below.
Nine Fundamental Mistakes in the Physics of Heat and in Greenhouse-Warming Theory Numerous assumptions central to greenhouse-warming theory turn out to be mistaken.
(1) Radiant energy cannot be quantified by a single number of watts per square meter as assumed in physics for more than two centuries. Planck’s empirical law shows clearly that radiation from matter, as a function of temperature, consists of a broad spectrum or continuum of frequencies of oscillation. According to the well-accepted Planck-Einstein relation, each frequency has its own energy. Therefore, energy radiated by a body of matter is a continuum of energies where all energies coexist. It makes no physical sense to integrate across these frequencies or energies to calculate a single number of watts per square meter. This approach seems to have worked adequately for most engineering applications where temperature differences are small. It fails catastrophically, however, for large differences in temperatures such as between Sun at 5500 oC and Earth at 15 oC.
(2) Radiative forcings cannot be added together. Temperature and heat are not additive. They are averative because heat flows by resonance.
(3) Earth’s surface temperature is not proportional to the sum of all radiative forcings. Planck’s empirical law shows clearly that temperature in matter is the result of a broad continuum of frequencies of oscillation and associated continuum of amplitudes of oscillation. Planck’s empirical law shows what frequencies and amplitudes of oscillation must be occurring throughout a body of matter for that body to possess a specific temperature.
(4) Greenhouse gases absorb only certain limited bands of frequencies of radiation emitted by Earth as shown in this diagram. Water is, by far, the strongest absorber, especially at lower frequencies.
Within these narrow frequency bands, what is being absorbed is only narrow spectral lines of oscillation shown in red in the diagram on the left. These spectral lines turn out to be the resonant frequencies of all the overtones of oscillation, of all the normal modes of oscillation or all the bonds holding the molecules together. Radiant energy is absorbed into the bonds, which has no direct effect on the temperature of the gas. Temperature of a gas is proportional to the average kinetic energy of translation of all atoms and molecules making up air. One has to assume that the absorbed oscillatory energy is converted by collisions into translational kinetic energy, a process that does not appear to be efficient.
Planck's law logarithmic frequencyÅngström (1900) showed that “no more than about 16 percent of earth’s radiation can be absorbed by atmospheric carbon dioxide, and secondly, that the total absorption is very little dependent on the changes in the atmospheric carbon dioxide content, as long as it is not smaller than 0.2 of the existing value.” Extensive modern data agree that carbon dioxide absorbs less than 16% of the frequencies emitted by Earth shown by the vertical black lines of this plot of Planck’s empirical law where frequencies are plotted on a logarithmic x-axis. These vertical black lines show frequencies and relative amplitudes only. Their absolute amplitudes on this plot are arbitrary.
Temperature at Earth’s surface is the result of the broad continuum of oscillations shown in green. Absorbing less than 16% of the frequencies emitted by Earth cannot have much effect on the temperature of anything.
(5) Greenhouse gases can only reradiate what they absorb. A molecule of carbon dioxide cannot physically emit black-body radiation. It can only emit the spectral lines that it absorbed into its bonds. When these limited spectral lines are absorbed by any body of matter, they would have minuscule effect on temperature of that body of matter because they only make up a very small part of the continuum of frequencies whose amplitudes of oscillation must be increased in order to warm a body of matter.
(6) Greenhouse gases do not reradiate in every direction as assumed. Radiation by resonance is observed only to flow from a hotter body of matter to a cooler body of matter, which at the molecular-bond scale is from a higher amplitude of oscillation to a lower amplitude of oscillation at the same frequency.
(7) Carbon dioxide makes up only 0.04% of the atoms and molecules in air. Any increase in energy resulting from absorption by carbon dioxide, must be shared with 2500 other molecules and atoms.
(8) Ångström (1900) shows by two experiments that greenhouse-gas concentrations of carbon dioxide in the atmosphere have minimal effect on air temperature. My experiments show that increasing carbon dioxide concentrations by a factor of more than 23 has no measurable effect on air temperature. Experiments reported on Internet that claim to see greenhouse warming utilize heat sources that are much, much hotter than Earth. More information at JustProveCO2.com.
(9) The thermal effects of radiation are not about amount of radiation absorbed, as currently assumed, they are about the temperature of the emitting body and the difference in temperature between the emitting and the absorbing bodies as explained above.
Greenhouse warming theory depends on at least nine assumptions that appear to be mistaken. Greenhouse warming theory has never been shown to be physically possible by experiment, a cornerstone of the scientific method. Greenhouse warming theory is rapidly becoming the most expensive mistake ever made in the history of science, economically, politically, and environmentally as explained in detail in sixteen short videos found at WhyClimateChanges.com/most-expensive-mistake/. Video 1 is an introduction. Videos 2 through 6 describe evidence for global warming and for the role of humans and of volcanic eruptions in causing observed climate change. Videos 7 through 10 explain what is mistaken concerning greenhouse-warming theory and why this theory is physically impossible. Videos 11 through 16 discuss issues related to setting informed public policy concerning climate change. A separate video, A Most Unexpected Revolution in the Physics of Heat, explains current problems with the physics of heat.
Click here for a 20-page scientific paper A Most Inconvenient Reality — Greenhouse Gases Cannot Physically Explain Observed Global Warming submitted to the Journal of Geophysical Research on May 28, 2018, that describes these issues in more detail. This file includes the editor’s email rejecting the paper without review and my response.
The thermal effects of radiation are not about amount of radiation absorbed, as currently assumed, they are about the temperature of the emitting body and the difference in temperature between the emitting and the absorbing bodies.
Heat is, in concept, what a body of matter must absorb to get warmer and emit to get cooler. Defining heat as thermal energy in transfer completely sidesteps the issue of what thermal energy is physically in matter and in radiation. Physics is supposed to be about what is physically happening in Nature. What physically is thermal energy? What physically is heat? Once we can answer these questions, then we can observe how, physically, thermal energy and heat flow through matter, through air, and through space?
What Physically Is Thermal Radiation?
Visible colors of sunlight separated by a prism.In 1672, Isaac Newton showed, using a prism and lenses, that sunlight is made up of a broad spectrum of colors and that these colors physically coexist within sunlight—they are displayed by but not created by the prism. Newton also showed that by using a second prism, he could combine the colors back into white light.
We observe that these colors do not interact with each other in any way in air and space—only when in the immediate presence of matter. We refer to these colors as the visible spectrum extending from red through violet, but they clearly form a continuous spectrum or continuum meaning a continuous sequence of shades of color in which adjacent colors are not perceptibly different from each other, although the extremes are quite distinct. The human eye can detect about ten million distinct colors.
Visible spectrumWe physically measure visible light as containing all frequencies of oscillation ranging from 450 to 789 terahertz, where one terahertz is one-trillion cycles per second (1012 cycles per second). We also observe that the visible spectrum is but a very small part of a much wider continuum that we call electromagnetic radiation Electromagnetic continuumwith frequencies extending over more than 20 orders of magnitude from extremely low frequency radio signals in cycles per second to microwave, infrared, visible, ultraviolet, X-rays, to gamma rays with frequencies of more than 100 million, million, million cycles per second (1020 cycles per second). Thermal radiation is a portion of this continuum of electromagnetic radiation radiated by a body of matter as a result of the body’s temperature—the hotter the body, shown here at the bottom as Temperature, the higher the radiated frequencies of oscillation with significant amplitudes of oscillation.
-
 4:13:23
4:13:23
What If Everything You Were Taught Was A Lie?
3 days agoDeclassified Government Documents "Open Your Eye Before You Die" Reveal America’s Dark Secrets
1.89K -
 LIVE
LIVE
Major League Fishing
4 days agoLIVE! - Bass Pro Tour: Stage 2 - Day 4
673 watching -
 56:24
56:24
Russell Brand
1 day agoEddie Gallagher: War, Betrayal & Fighting the System
83.1K11 -
 11:21
11:21
TimcastIRL
5 hours agoGOP Rep Says TWO SHOOTERS In JFK Assassination As FBI Uncovers TROVE Of Secret Documents
70.7K114 -
 1:04:55
1:04:55
Bare Knuckle Fighting Championship
4 days agoBKFC ITALY PRESS CONFERENCE | LIVE!
25.8K1 -
 10:04
10:04
Space Ice
3 hours agoThe Movie Silent Hill Is Like Resident Evil Without The Good Parts - Worst Movie Ever
14.1K3 -
 5:49
5:49
Hannah Barron
1 day agoRedneck Euro Mount
12.8K20 -
 32:34
32:34
hickok45
8 hours agoSunday Shoot-a-Round # 268
8.81K8 -
 27:33
27:33
The Finance Hub
18 hours ago $6.33 earnedBREAKING: ALINA HABBA JUST DROPPED A MASSIVE BOMBSHELL!!!
28.6K74 -
 40:23
40:23
PMG
22 hours ago $0.62 earnedHannah Faulkner and Dr. Michael Schwartz | EXPOSING BIG PHARMA
16.2K1