Premium Only Content
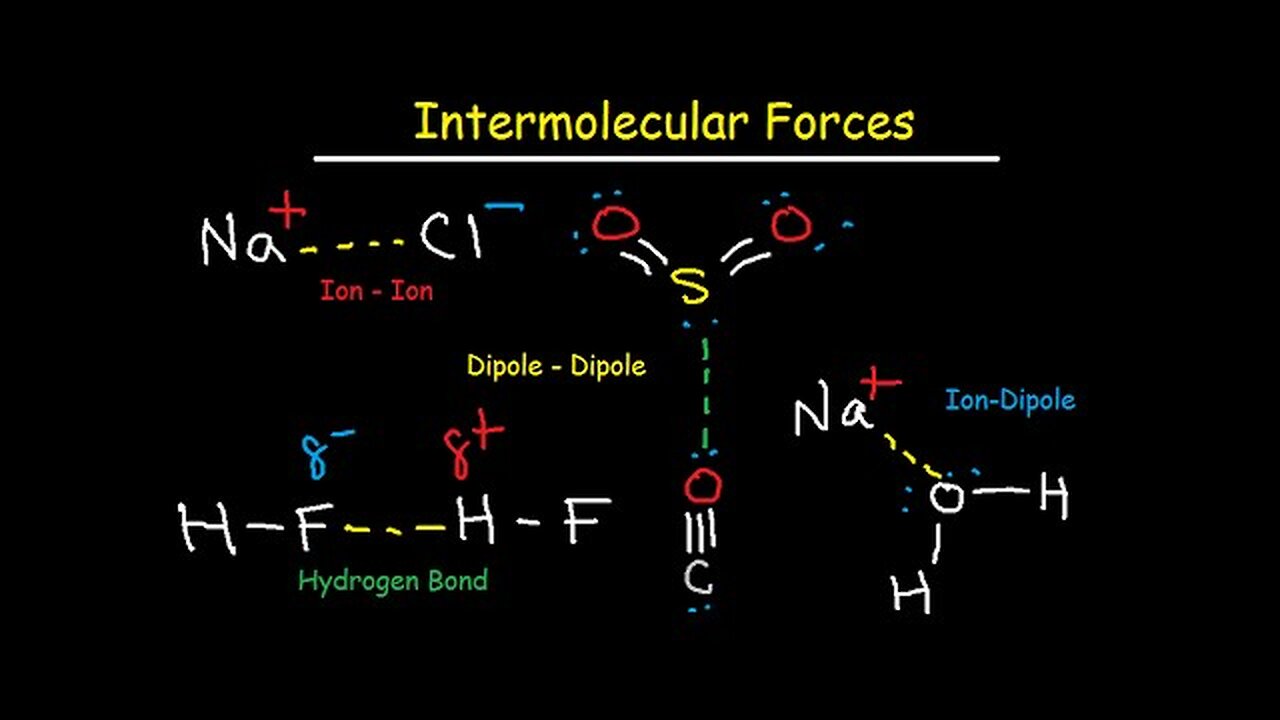
Intermolecular Forces - Hydrogen Bonding, Dipole-Dipole, Ion-Dipole, London Dispersion Interactions
This chemistry video tutorial focuses on intermolecular forces such hydrogen bonding, ion-ion interactions, dipole dipole, ion dipole, london dispersion forces and van deer waal forces. It contains plenty of examples and practice problems to help you understand the most important concepts related to this material.
Chemistry Basic Introduction and Final Exam Review:
https://www.video-tutor.net/chemistry...
My Twitter Page:
https://twitter.com/OrgoChemTutor21
Here is a list of topics:
1. Ion - Ion dipole interactions of KF and CaO
2. Electrostatic Force and Lattice Energy- The effect of charge and ionic radii or size
3. How To Determine Which Ionic Compound has a Higher Melting Point - NaF vs KCl
4. Ion-Dipole Interactions - NaCl and H2O
5. Definition of a Dipole - Polar Molecules & Charge Separation
6. Dipole-Dipole Interactions of Polar Molecules - Partial Charge Electrostatic Attractions of CO
7. Hydrogen Bonding between Hydrogen, Nitrogen, Oxygen, and Fluorine
8. Intermolecular Forces vs Intramolecular Forces
9. Hydrogen Bonding vs Polar & Nonpolar Covalent Bonds
10. London Dispersion Forces & Van Der Waals Forces
11. Permanent Dipoles and Temporary Induced Dipoles - Distribution of electrons in electron cloud
12. Difference Between Atoms and Ions - Cations vs Anions - Number of Electrons and Protons
13. The relationship between Polarizability and Dispersion Forces
14. How To Determine the Strongest Intermolecular Forces In Compounds Such as MgO, KCl, H2O, CH4, CO2, SO2, HF, CH3OH, LiCl, CH2O, CO, and I2
15. The relationship between Boiling Point and Vapor Pressure
16. Straight Chained vs Branched Alkanes - Boiling Point and Intermolecular Forces - Surface Area
17. Ranking Boiling Point In Order of Increasing Strength for I2, Br2, F2, and Cl2
18. Polar and Nonpolar Organic Compounds - Polarity and Water Solubility
19. Ranking Boiling In Decreasing Order For HF, HCl, HBr, and HI
20. The effect of Molar Mass and Number of electrons on the Overall Intermolecular Force / LDF
-
 1:04:05
1:04:05
TheOrganicChemistryTutor
19 days agoChemical Reactions - Combination, Decomposition, Combustion, Single & Double Displacement Chemistry
201 -
 LIVE
LIVE
Akademiks
1 hour agoASAP Rocky Case has Begun . He's facing 24 years for SH**ting his friend!
3,009 watching -
 LIVE
LIVE
Revenge of the Cis
2 hours agoEpisode 1436: Adios Amigo
1,713 watching -
 1:14:45
1:14:45
Awaken With JP
4 hours agoTrump's Making Everyone His B*tch LOL - LIES Ep 76
50.4K20 -
 1:41:43
1:41:43
Megyn Kelly
23 hours agoMedia's ICE Hoax, Vance's Masterclass on CBS, & Trump vs Bass, w/ Steve Bannon & Batya Ungar-Sargon
43.4K59 -
 1:04:28
1:04:28
In The Litter Box w/ Jewels & Catturd
22 hours agoGreen New Scam | In the Litter Box w/ Jewels & Catturd – Ep. 729 – 1/28/2025
55.3K29 -
 2:03:41
2:03:41
The Quartering
5 hours agoTrump To END Income Tax, Captain America Backlash, Obese Rapper Sues Lyft & Surge Pricing In Walmart
70.9K31 -
 1:05
1:05
tether
11 hours agoHadron by Tether - tokenize anything, anywhere
19.3K3 -
 34:32
34:32
Standpoint with Gabe Groisman
20 hours agoGovt. Workers AREN’T Working Says DOGE Chair Sen. Joni Ernst
15.3K9 -
 56:49
56:49
Savanah Hernandez
4 hours agoMASS DEPORTATIONS ARE HERE AND THEY ARE GLORIOUS
30.8K14