Premium Only Content

General Chemistry 1 Review Study Guide - IB, AP, & College Chem Final Exam
This video tutorial study guide review is for students who are taking their first semester of college general chemistry, IB, or AP Chemistry. Even if you’re studying for the general chemistry section of the MCAT, DAT, PCAT, OAT or SAT Subject chemistry test, this video can help give you a nice overview of all the topics you need to learn in General Chemistry 1. This introduction video contains plenty of examples and practice problems to help prepare you for the final exam. It has about 160 multiple choice questions in the form of a practice test. Feel free to use it as a study guide. The solutions to each problem is provided as well as the equations and formulas that you need to solve it.
Access The Full 8 Hour Video on Patreon:
https://www.patreon.com/MathScienceTutor
Direct Link to The Full Video:
https://bit.ly/3GstGjy
Download The Exam - 160 Practice Problems:
https://bit.ly/3VTpcbj
General Chemistry 2 Review - 7 Hours - 150 Practice Problems:
https://bit.ly/3X0t6AL
Youtube Membership:
/ @theorganicchemistrytutor
General Chemistry 2 Final Exam Review:
• General Chemistry 2 Review Study Guid...
Here is a list of topics:
1. How To Find The Number of Protons, Neutrons, and Electrons In an Atom
2. Nomenclature of Ionic and Molecular Compounds
3. Percent Composition, Stoichiometry, Molarity, Dilution Problems, Acid Base Titrations
4. Oxidation State, Combined Gas Law, Gas Density at STP, Dalton’s Law of Partial Pressure
5. Collected over water gas law problems, How to Identify the Unknown Compound
6. Average Kinetic Energy of Gases, Real Gas vs Ideal Gas High Temperature Low Pressure
7. Specific Heat Capacity, Heat of Fusion, Heat of Combustion, Enthalpy, Products – Reactants,
8. Condensation, Sublimation, Melting, Vaporization, Freezing, Deposition
9. Heat of Formation, Wavelength Frequency and Energy of a photon, Electron Configuration
10. Which four quantum numbers are incorrect? N l ml ms, s p d f orbitals,
11. Electromagnetic radiation – Radio waves Microwaves Infrared Visible Light Ultraviolet X-rays and Gamma rays.
12. Ionization Energy Electronegativity Atomic Radii Ionic Radius Electron Affinity and Metallic Character
13. Hybridization, Molecular Geometry, Lewis Structure, Bond Angle, Polar or Nonpolar
14. SO2, CO2, CH4, BH3, H2O, NH3, BF3, SF6, NF3, SF4, PCl5, XeF4
15. Intermolecular Forces, Hydrogen Bonding, Dipole Dipole Interactions, London Dispersion Forces, Ion Dipole, Permanent Dipoles, Temporary Induce Dipole, Van der Waal Forces, and Ionic Bonds.
16. Highest Boiling Point, Lowest Freezing Point, pH of a solution, Empirical Formula,
17. Molality, Mass Percent, Density, Solutions, Nonelectrolytes, Vapor Pressure, Combustion Analysis
18. Colligative Properties – Boiling Point Elevation, Freezing Point Depression, Osmotic Pressure
19. Average atomic mass and Percent Relative Abundance, Chemical Change, Chemical Formula
20. Actual Yield, Theoretical Yield, and Percent Yield, Oxidizing Agent vs Reducing Agent
21. Grams to moles to molecules to atoms – conversions / dimensional analysis
22. How To Find The Mass of The Excess Reactant That Remains, Limiting Reactants,
23. Homogeneous Mixture – Air plus salt and water, Soluble vs Insoluble
24. Representative Elements, Transition Metals, Alkali Metals, Alkaline Earth Metals, Halogens, Chalcogens, Noble gases, and Inner Transition Metals
25. Redox Reactions, Single Replacement Reactions, Combustion Reactions, Synthesis Reactions, Combination Reactions, and Decomposition Reactions, Double Replacement Reactions
26. Acid Base Neutralization Reactions, Precipitation Reactions, Gas Evolution Reactions
27. How to Identify the Unknown Metal given its Oxide and Formula, Gas Stoichiometry
28. Ideal Gas Law PV=nRT, Molar mass of unknown gas, mole fraction and partial pressure,
29. Root Mean Square Velocity, Graham’s Law – Rate of Effusion, Kinetic Molecular Theory
30. Pressure Volume Temperature Graphs – Boyle’s Law, Charles Law, and Gay Lussac’s Law
31. ionic and covalent bonds, Internal Energy of System,
32. Final Temperature of Water Solution, Hess Law, Thermochemical Equations,
33. Endothermic vs Exothermic, Calorimetry, Lattice Energy, Valence Electrons, Paramagnetic.
34. electron configuration exceptions, noble gas notation
35. Hund’s rule, Heisenberg Uncertainty Principle, Aufbau Principle, Pauli Exclusion Principle
Disclaimer: Some of the links associated with this video may generate affiliate commissions on my behalf. As an amazon associate, I earn from qualifying purchases that you may make through such affiliate links.
-
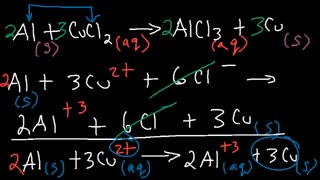 20:32
20:32
TheOrganicChemistryTutor
2 months agoSingle Replacement Reactions and Net Ionic Equations
251 -
 46:08
46:08
Kimberly Guilfoyle
16 hours agoBad Day to be a Bad Guy: FBI Taking Down World’s Worst Criminals, Live with John Nantz | Ep.203
204K85 -
 DVR
DVR
Redacted News
15 hours agoWhat's REALLY going on in Syria? | Redacted with Natali Morris
203K135 -
 54:18
54:18
Candace Show Podcast
15 hours agoHarvey Speaks: Jessica Mann & The Five Year Affair | Ep 3
221K120 -
 56:53
56:53
Grant Stinchfield
14 hours ago $10.19 earnedFreeze Spending & Kick the Can Down the Road... Why Republicans Should do Just That!
120K25 -
 56:48
56:48
VSiNLive
14 hours agoFollow the Money with Mitch Moss & Pauly Howard | Hour 1
91.9K1 -
 3:28:27
3:28:27
Barry Cunningham
15 hours agoTRUMP DAILY BRIEFING: INTERNET UNDER ATTACK! X & RUMBLE DOWN! EXECUTIVE ORDER SIGNING!
107K63 -
 5:53:56
5:53:56
Scammer Payback
18 hours agoCalling Scammers Live
92.2K8 -
 1:36:15
1:36:15
In The Litter Box w/ Jewels & Catturd
1 day agoABOLISH NGOs | In the Litter Box w/ Jewels & Catturd – Ep. 758 – 3/10/2025
108K64 -
 2:04:36
2:04:36
Film Threat
1 day agoVERSUS: DAREDEVIL: BORN AGAIN + MICKEY 17 + THE STATE OF SCI-FI | Film Threat Versus
51.7K2