Premium Only Content
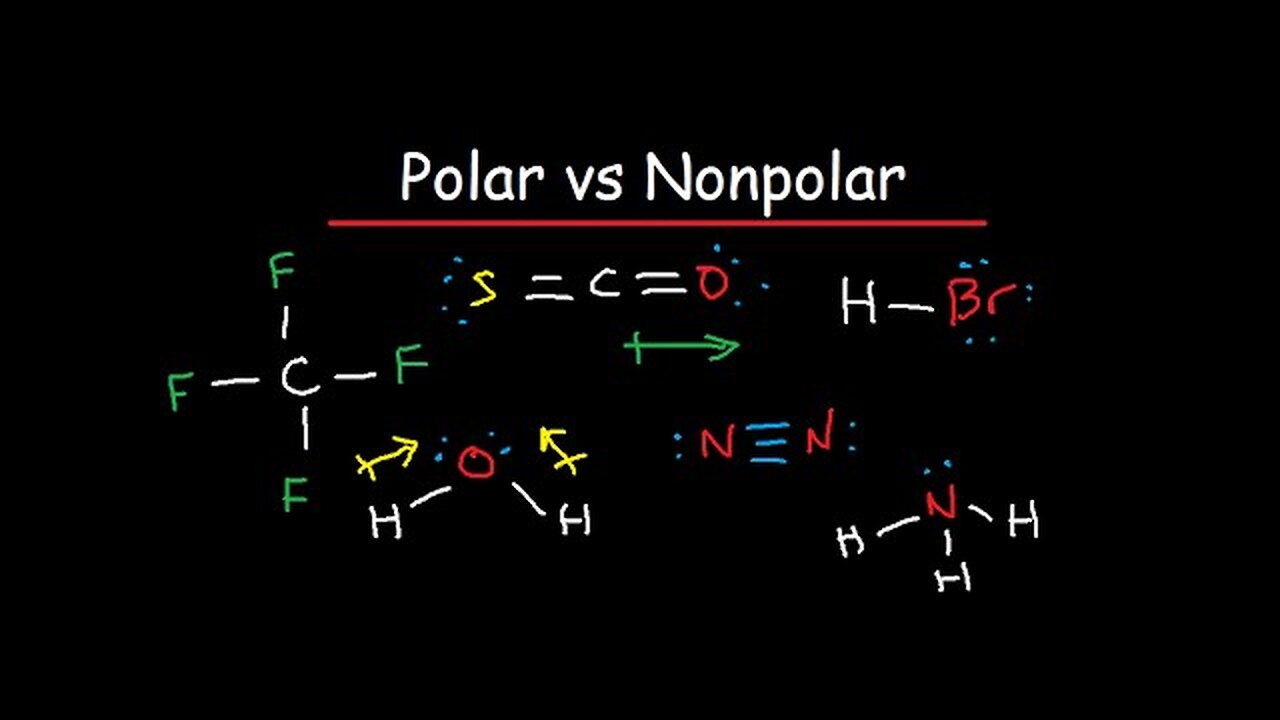
Polar and Nonpolar Molecules: Is it Polar or Nonpolar?
This video discusses how to tell if a molecule / compound is polar or nonpolar. Here is a list of molecules that are considered.
My E-Book: https://amzn.to/3B9c08z
Video Playlists: https://www.video-tutor.net
Homework Help: https://bit.ly/Find-A-Tutor
Subscribe: https://bit.ly/37WGgXl
Support & Donations: https://www.patreon.com/MathScienceTutor
Youtube Membership:
/ @theorganicchemistrytutor
General Chemistry Video Playlist:
• Intro to Chemistry, Basic Concepts - ...
Nonpolar Molecules:
Diatomic Molecules: H2, N2, O2, Cl2, Br2, F2, I2
Hydrocarbons: CH4, C2H6, C3H8, C2H2, C2H4
Identical Outer Elements With No Lone Pair on Central Atom:
Tetrahedral Molecular Geometry:
SiBr4, CCl4, CF4, GeH4, CBr4, SiH4
Trigonal Bipyramidal Molecular Geometry:
PCl5, PF5, AsF5, PBr5, SbCl5
Linear Molecular Geometry:
CO2, CS2, BeH2, BeCl2, and BeF2
Trigonal Planar Molecular Geometry:
BH3, AlCl3, AlBr3, AlF3, FeBr3
Octahedral Molecular Geometry:
SeF6, SBr6, SF6, SeCl6, SI6, SeI6
Polar Molecules:
Same Outer Element With an Assymetrical Lone Pair(s)
Bent Molecular Geometry:
H2S, H2O, H2Se, SF2, SCl2, SeBr2, SO2, SeO2
Trigonal Pyramidal Molecular Geometry:
NH3, PH3, PBr3, PCl3, NF3
T-shaped Molecular Geometry:
IF3, ClF3, BrF3, ICl3, BrCl3
Square Pyramidal Molecular Geometry:
IF5, ClF5, BrF5, ICl5, BrCl5
SeeSaw Molecular Geometry:
SF4, SeCl4, SBr4, SeI4
Exception: XeF4
Different Outer Elements: (Usually Polar)
CH3F, CSO, BH2F
Disclaimer: Some of the links associated with this video may generate affiliate commissions on my behalf. As an amazon associate, I earn from qualifying purchases that you may make through such affiliate links.
-
 13:10
13:10
TheOrganicChemistryTutor
3 days agoDependent and Independent Variables
44 -
 2:03:46
2:03:46
LFA TV
17 hours agoTIME FOR A NEW SPEAKER! | LIVE FROM AMERICA 12.26.24 11am EST
49.3K30 -
 1:40:22
1:40:22
Game On!
14 hours ago $4.74 earnedNFL Thursday Night Football Seahawks at Bears EXPERT Picks!
39.7K9 -
 1:50:54
1:50:54
xBuRnTx
3 hours agoWho's Ready for New Years!
29.2K1 -
 12:09
12:09
Tactical Advisor
16 hours agoSmith & Wesson Shield Plus Carry Comp
23.8K1 -
 4:35:25
4:35:25
Father Russell
9 hours agoDelta Force | Not A Woman? | Mad Martigan Time
64.7K5 -
 3:29:42
3:29:42
BrookieMonster
16 hours ago $44.71 earnedChristmas Stream: Marvel Rivals with CallmeSeags 🎄
183K13 -
 LIVE
LIVE
TheSaf3Hav3n
4 days ago| RUMBLES FIRST SUBATHON IS HERE!!! | DAY 4 |
287 watching -
 6:54
6:54
Dr. Nick Zyrowski
4 hours agoHIGH PROTEIN DIET Fixes Your Metabolism! - Weight Loss Not Required
19.4K5 -
 3:14:33
3:14:33
Joe Donuts Gaming
18 hours ago🟢 Live : Christmas is Here!! | Fortnite, Caroling, Light Tours and Donos !!
95.6K15