Premium Only Content
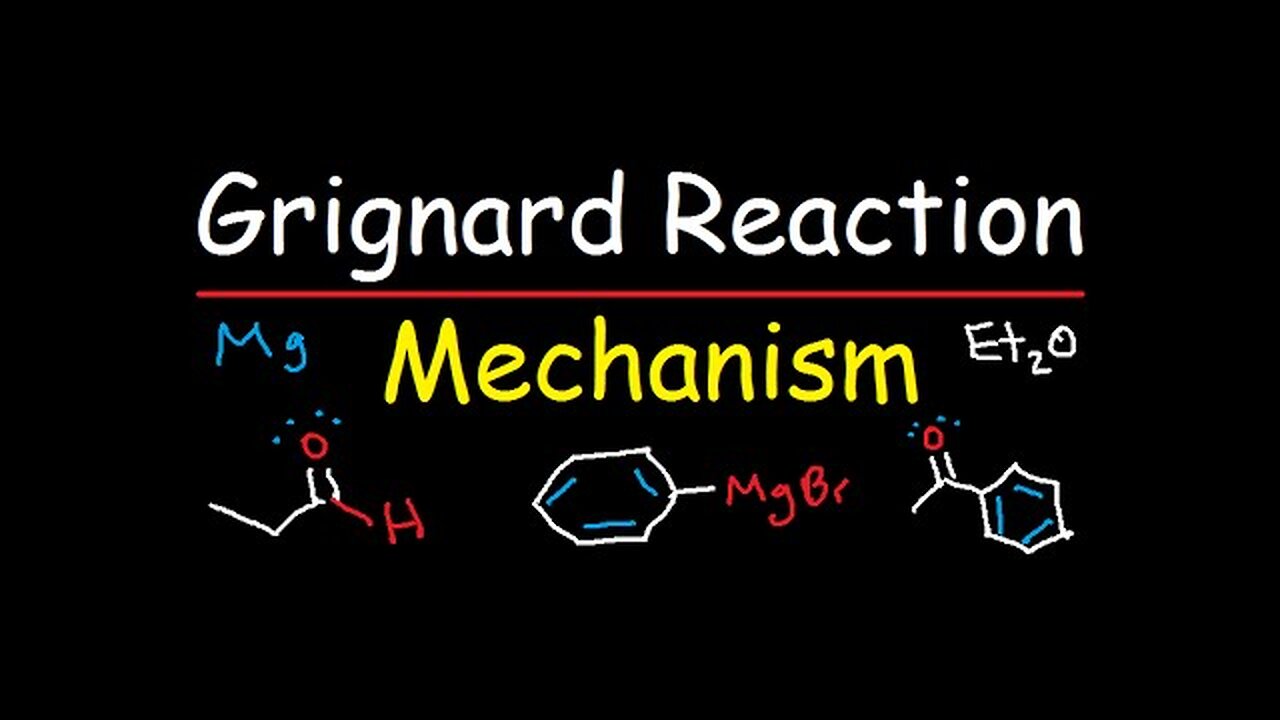
Grignard Reagent Synthesis Reaction Mechanism - Organic Chemistry
This organic chemistry video tutorial discusses the synthesis reaction mechanism of grignard reagents with water - H2O, D2O, aldehyes including formaldehyde, ketones, esters, acid chlorides, epoxides - ethylene oxide, CO2, etc. This video contains examples and practice problems that not only help you to predict the major product of the reaction but also how to choose the right reagents to synthesize a primary, secondary, and tertiary alcohol. In addition, other reactions were included in this video such as oxidation by H2CrO4, PCC, KMnO4 & H3O+, in addition to Na2Cr2O7 with H2SO4. Reduction of an alkyne to a cis alkene using H2 & lindlar's catalyst and to a trans alkene using Na and NH3 were also considered. The conversion of an alcohol into an alkyl halide using SOCl2 and PBr3 were also mentioned. The reagents used in this video include phenyl magnesium bromide, methyl magnesium bromide, ethyl magnesium bromide, acetyl chloride, cyclopentyl magnesium bromide, acetaldehyde, 2-butanone, etc.
-
 2:00:36
2:00:36
TheOrganicChemistryTutor
1 month agoSeries and Parallel Circuits Explained - Voltage Current Resistance Physics - AC vs DC & Ohm's Law
31 -
 12:03
12:03
Space Ice
16 hours agoSteven Seagal's China Salesman - Mike Tyson Knocks Him Out - Worst Movie Ever
44.6K16 -
 11:37
11:37
Degenerate Jay
16 hours ago $14.75 earnedJames Bond Needs Quality Over Quantity From Amazon
92K8 -
 15:23
15:23
Misha Petrov
16 hours agoTrad Wives & Girl Bosses Go to WAR!
70.5K46 -
 2:03:11
2:03:11
TheDozenPodcast
14 hours agoFootball villain fighting the state: Joey Barton
55.1K1 -
 13:18:50
13:18:50
Scottish Viking Gaming
17 hours ago💚Rumble :|: Sunday Funday :|: Smash the Blerps and Vape the Terpes
93.3K8 -
 1:45:00
1:45:00
RG_GerkClan
19 hours ago🔴LIVE Sunday Special - It's Time for World Domination - Civilization VII - Gerk Clan
86.9K28 -
 LIVE
LIVE
Major League Fishing
4 days agoLIVE Tackle Warehouse Invitationals, Stop 1, Day 3
97 watching -
 23:34
23:34
marcushouse
19 hours ago $16.14 earnedBREAKING: Starship Launch IMMINENT – But What’s This SURPRISE Flight 9 Plan?! 🚀🔥
123K18 -
 8:43
8:43
Film Threat
1 day agoTHE MONKEY | Film Threat Reviews
109K4