Premium Only Content
Inductive Effect | Types of Inductive Effect| Application of Inductive Effect|2023 | AIM Pharma
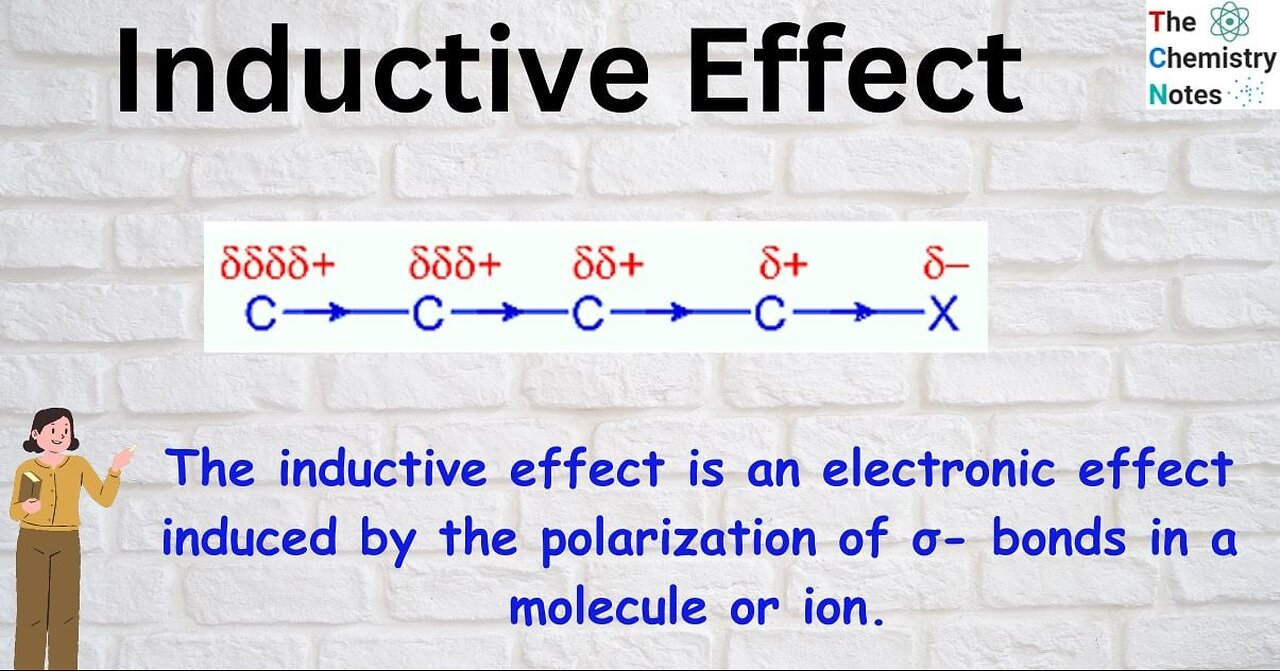
In chemistry, the inductive effect in a molecule is a local change in the electron density due to electron-withdrawing or electron-donating groups elsewhere in the molecule, resulting in a permanent dipole in a bond.[1] It is present in a σ (sigma) bond, unlike the electromeric effect which is present in a π (pi) bond.
The halogen atoms in an alkyl halide are electron withdrawing while the alkyl groups have electron donating tendencies. If the electronegative atom (missing an electron, thus having a positive charge) is then joined to a chain of atoms, usually carbon, the positive charge is relayed to the other atoms in the chain. This is the electron-withdrawing inductive effect, also known as the -I effect. In short, alkyl groups tend to donate electrons, leading to the +I effect. Its experimental basis is the ionization constant. It is distinct from and often opposite to the mesomeric effect
The effect of the sigma electron displacement towards the more electronegative atom by which one end becomes positively charged and the other end negatively charged is known as the inductive effect. The -I effect is a permanent effect & generally represented by an arrow on the bond.[citation needed]
However, some groups, such as the alkyl group, are less electron-withdrawing than hydrogen and are therefore considered as electron-releasing/ electron-donating groups. This is electron-releasing character and is indicated by the +I effect. In short, alkyl groups tend to give electrons, leading to the induction effect. However, such an effect has been questioned.[2]
As the induced change in polarity is less than the original polarity, the inductive effect rapidly dies out and is significant only over a short distance. Moreover, the inductive effect is permanent but feeble since it involves the shift of strongly held σ-bond electrons and other stronger factors may overshadow this effect. #inductiveeffect #youtubeshorts #positiveinductive effects
#negative inductive effects
#chemistry #2023 #health #medicine #AIM Pharma
-
 1:27:59
1:27:59
Kim Iversen
9 hours agoRFK Jr Declares No More Cheetos on Welfare? | Yale Confirms Long Covid Is Actually Vaccine Injury!
72K99 -
 1:08:43
1:08:43
The Charlie Kirk Show
6 hours agoTHOUGHTCRIME Ep. 74 — Charlie's Campus Return? Robo-Butlers? Garden of American Heroes?
67.8K17 -
 1:09:53
1:09:53
Slightly Offensive
6 hours ago $5.29 earnedIs the US Headed for MORE WAR Under TRUMP? | Guest: Scott Horton
36.8K9 -
 58:29
58:29
The StoneZONE with Roger Stone
6 hours agoRoger Stone Hails Confirmation of Kash Patel, Trashes Schiff for Attacks On Patel | The StoneZONE
43.8K17 -
 48:44
48:44
Man in America
11 hours agoA MASSIVE Global Financial Reset Is Coming—Are You Ready?
32.9K7 -
 1:15:42
1:15:42
Precision Rifle Network
1 day agoS4E5 Guns & Grub - The Best Rifle Under $2000
48.6K6 -
 1:02:54
1:02:54
Glenn Greenwald
1 day agoSouth Korean Economist Ha-Joon Chang on the Economic World Order, Trump's Tariffs, China & More | SYSTEM UPDATE #410
77.4K46 -
 1:02:27
1:02:27
Donald Trump Jr.
11 hours agoBye Mitch, plus Kash confirmed, Interview with AJ Rice | Triggered Ep.218
126K73 -
 1:12:27
1:12:27
The Amber May Show
13 hours ago $2.90 earnedWomen Of Rumble 02-20-25
33.2K7 -
 41:18
41:18
Kimberly Guilfoyle
11 hours agoToday, We Kash in on Equal Justice, Live with Ryan Walters & Daniel Turner | Ep.198
96.9K22