Premium Only Content
DEFEND YOUR HEALTH CANADA - Say NO to the Agile Licensing Amendments to our Food & Drug Act
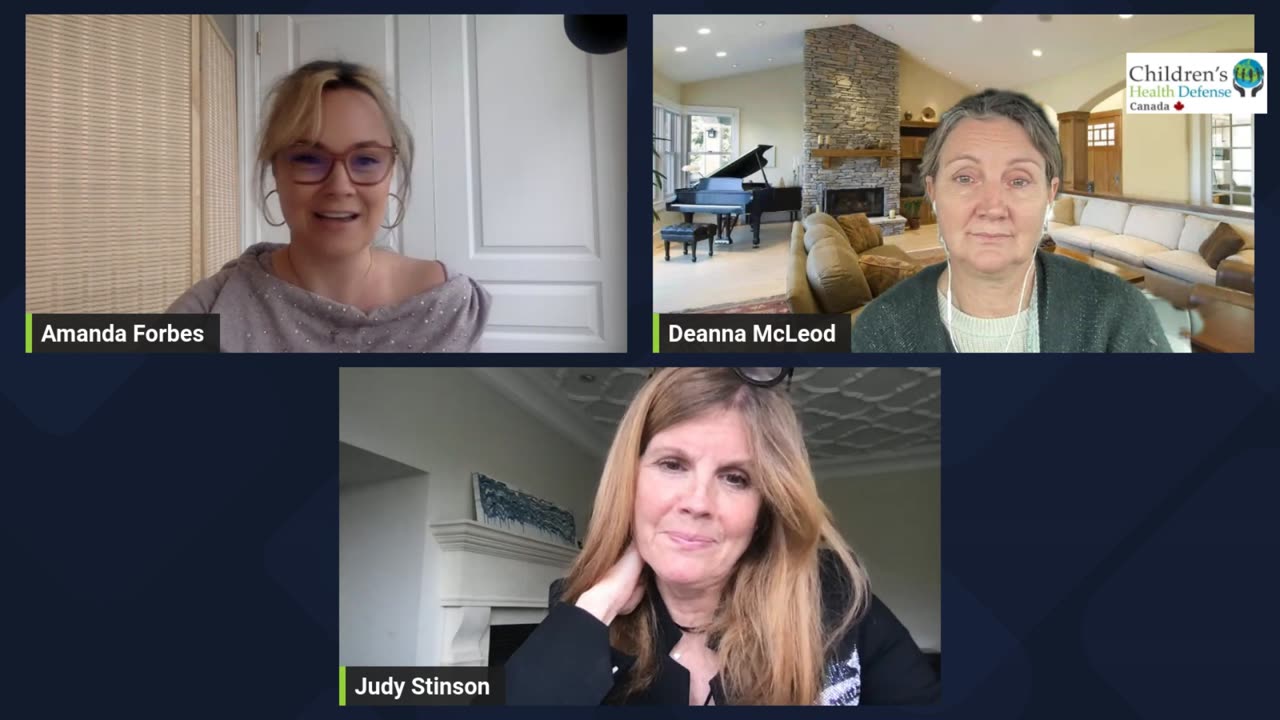
Amanda Forbes welcomes Deanna McLeod and Judy Stinson from the Canadian Covid Care Alliance for a discussion that Shines a Light on the "Advanced Therapeutics Products" Pathway, an action that is being pushed through by the Canadian Health Minister.
In a backdoor effort to allow Pharma and other big agencies to come in and re-write the current amendments that will LOWER regulatory standards and would fast track potentially high risk therapies without requiring they first be PROVEN SAFE. These companies are only simply required to argue that the benefits outweigh the risks and that the risks can be "managed" rather than PROVING safety for our Canadian Public.
ACTION:
PLEASE take 5 minutes to express the Concerns you have for this to Health Canada
*Submit a general Comment about 'Agile Licensing" at the top of the Canada Gazette Webpage by APRIL 26
*Send that same comment to :
BRUNO RODRIGUE, Executive Director, Office of Legislative & Regulatory Modernization, Health Products & Food Branch, Department of Health, 3000A, 11 Holland Ave, Suite P2108, Ottawa, Ontario K1A 0K9
(email: LRM.consultations-mlr@hc-sc.gc.ca)
*Contact your MP to express concern about 'Agile Licensing"
DEMAND an immediate re-examination of ALL Agile Licensing Regulations & proposed amendments, and demand an investigation into the individuals involved in creating this industry favouring backdoor that places Canadians at risk of product harm by rushing novel products to the market without first proving their safety & efficacy.
*Find your member of Parliament here: https://www.ourcommons.ca/members/en/search
If you find value in our work, please consider donating to ChildrensHealthDefense.ca
-
 55:40
55:40
Children's Health Defense Canada
1 year agoDr. Peter McCullough's #1 Advice STOP Getting the Shots
9495 -
 LIVE
LIVE
WeAreChange
2 hours agoBREAKING: Biden Admin Could SEND NUKES To Ukraine?! UK & France To Send Troops? w/ Roger Stone
1,603 watching -
 1:35:49
1:35:49
The Officer Tatum
2 hours agoLIVE: Jack Smith DROPS Case, Elon Musk BREAKS MSNBC | OT Show EP 14
26.2K18 -
 45:24
45:24
Kimberly Guilfoyle
5 hours agoAmericans Cheer Trump Cabinet Picks, Live with Dr Ben Carson & Mike Davis | Ep. 177
51.3K11 -
 48:28
48:28
Roseanne Barr
2 hours ago $7.77 earnedBonus Episode!!! with Noble Gold CEO Collin Plume | The Roseanne Barr Podcast
37.4K67 -
 LIVE
LIVE
LFA TV
20 hours agoMorning Joke | Trumpet Daily 11.25.24 7PM EST
146 watching -
 LIVE
LIVE
Sarah Westall
1 hour agoPreparing Humanity for the Next Age: Harmonizing Body Frequencies & Military Grade Advanced Tech
95 watching -
 LIVE
LIVE
2 MIKES LIVE
1 hour ago2 MIKES LIVE #147 Breaking News!
86 watching -
 LIVE
LIVE
VOPUSARADIO
2 days agoThe Dan Smeriglio Show: Special Guest- Veteran, Rapper & TPUSA Ambassador TOPHER!
24 watching -
 49:23
49:23
Exploring With Nug
9 hours agoWoman Disappears From L.A. and How Her Father Is Found Dead!
6.14K1