Premium Only Content
An Introduction To Plant Breeding-Teaching Us about Preparing for a Plant Pandemic?
Plant breeding is a technique through which genetic traits of a plant are changed. Some desirable traits are incorporated to produce a new variety. This technique is mainly used to improve the quality of food crop, to produce high yielding crop varieties and also provide resistance to diseases and pests. Teach Us about Preparing for a Plant Pandemic ? The question is not whether we’ll experience such an event; it’s whether we’ll be ready when it strikes As the world continues to fight COVID-19, the menace of infectious disease has never been more apparent. The next devastating pandemic could strike plants. Agricultural pathogens are evolving and spreading at a troubling rate—and the COVID pandemic offers important lessons for how we should prepare for them.
Plant diseases can be catastrophic. One of the worst was Panama disease, which destroyed banana plantations in Central and South America in the 1950s, devastating a critical food source and industry. Panama Disease is caused by a fungus, and, like most fungi, it spreads through spores. Those microscopic particles are carried by the wind, rain and soil—in this case, all the way from Panama throughout Central America and into South America.
Spores spread easily by their nature, but global trade and climate change are accelerating this process. Powerful storms and other extreme weather events bring pathogens to new regions where plants haven’t developed resistance. Modern monoculture farming only increases crops’ vulnerability to infection.
Effective solutions exist to control plant diseases: chemical fungicides, resistant crop varieties and the emerging use of biological pesticides. The company where I work, Joyn Bio, is in the business of biologicals, where we engineer naturally occurring microbes to create high performance biopesticides and biofertility products.
However, just like SARS-CoV-2, plant diseases mutate quickly, and variants require new control strategies. For example, Panama disease was initially defeated by the introduction of the now-familiar Cavendish banana variety, which was resistant. However, through mutation, a variant of the disease can now infect these plants and threatens commercial banana production globally.
To protect plants against breakthrough pathogens, we need to prepare. The past years have unfortunately shown us the importance of such preparation.
While it’s not yet possible to vaccinate plants like we vaccinate humans, we can learn from the success of COVID-19 vaccine development, which happened in record time by building upon a solid scientific foundation. Moderna produced its vaccine prototype days after accessing the viral genome sequence—an achievement that was possible only because of existing research in immunology and virology, coupled with new technologies for vaccine development, like mRNA vaccines.
In agriculture, we have effective surveillance systems to warn us of emerging agricultural pathogens, but we lack the framework to rapidly develop solutions to those threats. We need to build the technical framework now, because once in the throes of a pandemic, it will be too late.
A new framework could draw on programs to accelerate the development of human antibiotics; because of the evolution of antibiotic resistance in bacteria, novel treatments are necessary, and our best bet is to identify threatening “superbugs,” then prototype new antibiotics before those strains break out.
In the agricultural world, mega-threats are mostly fungi, which are responsible for the majority of plant diseases. Existing fungicides and plant-breeding techniques are effective and widely used, but in the event of a breakthrough, we’re in trouble. It can require a decade or more to develop new chemical agents or plant-breeding solutions.
In the interim, there are opportunities to build upon progress in synthetic biology to advance biofungicides. At Joyn Bio we are working on new fungicidal modes of action and microbes to deliver these to crop plants. Biofungicides encompass a broad category of solutions ranging from live microbes to biological chemistries like proteins and nucleic acids. We can “preload” the discovery and development of new treatments in this category using proven biotech tools.
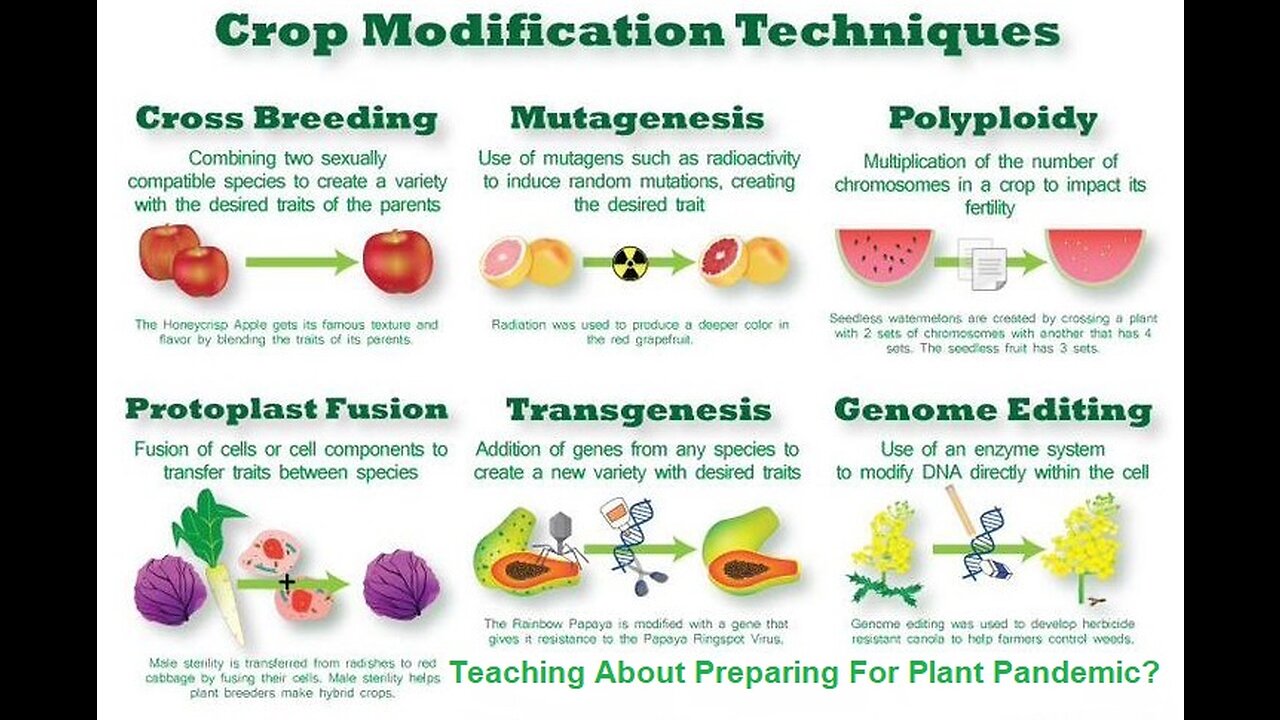
GMO Technologies in Plant Breeding GMO plants, also called transgenic plants, refer to plants that are altered by scientists using genetic engineering methods. A traditional and typical technique to create GMO plants involves several steps (Fig. 1): 1) choosing and isolating the coding sequence of a desirable trait gene; 2) constructing a fusion gene vector using the coding sequence and other necessary DNA elements such as promoter and terminator sequences, and also a selectable marker gene; 3) inserting the trait gene and selectable marker gene into a host plant genome. Inserting genes into the genome of a host plant can be accomplished with Agrobacterium- or biolistics-(‘gene gun’) mediated transformation. For Agrobacterium-mediated plant transformation, the T-DNA plasmid of Agrobacterium is the vector that hosts the trait gene and selectable marker gene. Agrobacterium containing the T-DNA plasmid can help insert these genes into the genome of a host plant that is susceptible to Agrobacterium infection. For the biolistic transformation, small gold or tungsten particles are coated with DNA of the trait gene and selectable marker gene and then bombarded into plant cells. Some DNA entering the nucleus can be integrated into the host plant genome. The biolistic method can be used on plants that are not susceptible to Agrobacterium infection. The following is an updated summary of the FDA’s Inventory of Completed Biotechnology Consultations on GMO plants, available to the public:
Science and History of GMOs and Other Food Modification Processes How has genetic engineering changed plant and animal breeding?
For thousands of years, humans have been using traditional modification methods like selective breeding and cross-breeding to breed plants and animals with more desirable traits. For example, early farmers developed cross-breeding methods to grow corn with a range of colors, sizes, and uses. Today’s strawberries are a cross between a strawberry species native to North America and a strawberry species native to South America.
Most of the foods we eat today were created through traditional breeding methods. But changing plants and animals through traditional breeding can take a long time, and it is difficult to make very specific changes. After scientists developed genetic engineering in the 1970s, they were able to make similar changes in a more specific way and in a shorter amount of time.
A Timeline of Genetic Modification in Agriculture Circa
8000 BCE: Humans use traditional modification methods like selective breeding and cross-breeding to breed plants and animals with more desirable traits.
1866: Gregor Mendel, an Austrian monk, breeds two different types of peas and identifies the basic process of genetics.
1922: The first hybrid corn is produced and sold commercially.
1940: Plant breeders learn to use radiation or chemicals to randomly change an organism’s DNA.
1953: Building on the discoveries of chemist Rosalind Franklin, scientists James Watson and Francis Crick identify the structure of DNA.
1973: Biochemists Herbert Boyer and Stanley Cohen develop genetic engineering by inserting DNA from one bacteria into another.
1982: FDA approves the first consumer GMO product developed through genetic engineering: human insulin to treat diabetes.
1986: The federal government establishes the Coordinated Framework for the Regulation of Biotechnology. This policy describes how the U.S. Food and Drug Administration (FDA), U.S. Environmental Protection Agency (EPA), and U.S. Department of Agriculture (USDA) work together to regulate the safety of GMOs.
1992: FDA policy states that foods from GMO plants must meet the same requirements, including the same safety standards, as foods derived from traditionally bred plants.
1994: The first GMO produce created through genetic engineering—a GMO tomato—becomes available for sale after studies evaluated by federal agencies proved it to be as safe as traditionally bred tomatoes.
1990s: The first wave of GMO produce created through genetic engineering becomes available to consumers: summer squash, soybeans, cotton, corn, papayas, tomatoes, potatoes, and canola. Not all are still available for sale.
2003: The World Health Organization (WHO) and the Food and Agriculture Organization (FAO) of the United Nations develop international guidelines and standards to determine the safety of GMO foods.
2005: GMO alfalfa and sugar beets are available for sale in the United States.
2015: FDA approves an application for the first genetic modification in an animal for use as food, a genetically engineered salmon.
2016: Congress passes a law requiring labeling for some foods produced through genetic engineering and uses the term “bioengineered,” which will start to appear on some foods.
2017: GMO apples are available for sale in the U.S.
2019: FDA completes consultation on first food from a genome edited plant.
2020: GMO pink pineapple is available to U.S. consumers.
2020: Application for GalSafe pig was approved.
How are GMOs made?
“GMO” (genetically modified organism) has become the common term consumers and popular media use to describe foods that have been created through genetic engineering. Genetic engineering is a process that involves:
Identifying the genetic information—or “gene”—that gives an organism (plant, animal, or microorganism) a desired trait
Copying that information from the organism that has the trait
Inserting that information into the DNA of another organism
Then growing the new organism
How Are GMOs Made? Fact Sheet
Making a GMO Plant, Step by Step
The following example gives a general idea of the steps it takes to create a GMO plant. This example uses a type of insect-resistant corn called “Bt corn.” Keep in mind that the processes for creating a GMO plant, animal, or microorganism may be different.
Making a GMO Plant, Step by Step - Identify
Identify
To produce a GMO plant, scientists first identify what trait they want that plant to have, such as resistance to drought, herbicides, or insects. Then, they find an organism (plant, animal, or microorganism) that already has that trait within its genes. In this example, scientists wanted to create insect-resistant corn to reduce the need to spray pesticides. They identified a gene in a soil bacterium called Bacillus thuringiensis (Bt), which produces a natural insecticide that has been in use for many years in traditional and organic agriculture.
Making a GMO Plant, Step by Step - Copy
Copy
After scientists find the gene with the desired trait, they copy that gene.
For Bt corn, they copied the gene in Bt that would provide the insect-resistance trait.
Making a GMO Plant, Step by Step - Insert
Insert
Next, scientists use tools to insert the gene into the DNA of the plant. By inserting the Bt gene into the DNA of the corn plant, scientists gave it the insect resistance trait.
This new trait does not change the other existing traits.
Making a GMO Plant, Step by Step - Grow
Grow
In the laboratory, scientists grow the new corn plant to ensure it has adopted the desired trait (insect resistance). If successful, scientists first grow and monitor the new corn plant (now called Bt corn because it contains a gene from Bacillus thuringiensis) in greenhouses and then in small field tests before moving it into larger field tests. GMO plants go through in-depth review and tests before they are ready to be sold to farmers. The entire process of bringing a GMO plant to the marketplace takes several years.
Vaccines are the most cost-effective and efficacious means of reducing the disease burden of infectious diseases. Novel approaches to improve product quality and improve access while lowering production costs are continuously being sought. Traditionally vaccines are prepared by using an attenuated version of the pathogen or by preparing and inactivating a disease-causing organism or a suitable part of it, e.g. a toxin, and administering it in quantities sufficient to induce immunity. Such vaccines have the unavoidable potential for contamination with adventitious agents that can infect such materials. A new and exciting possibility is the production of vaccine antigens in genetically modified plants which can then be extracted and purified by conventional methods. Such vaccines could either be eaten or applied to mucosal surfaces.
Plant-derived vaccines have several advantages. They can be produced cheaply in very high amounts, carrier plants such as potatoes and corn are readily accepted by patients and antigens derived from them are stable and can be stored for long periods of time. The likelihood that contamination by a plant virus would have an adverse effect on humans is almost negligible. There are several technical challenges concerning plant-derived vaccines that must be resolved before they can enter wide-scale use and the regulatory requirements for this novel class of vaccines must be established. In addition, public acceptance of the new technology must be ensured. As the development of plant-derived vaccines matures, WHO will continue to serve as a forum for the international harmonization of requirements.
Genetic Engineering and Plant Protection Genetic engineering can be used in a variety of ways to protect plants from damaging pests and diseases. Why is it important to protect plants from pests and diseases? In commercial agriculture, plants are typically grown in genetic monocultures, especially staple crops like corn, wheat, rice and others. If a pest or pathogen is present or introduced and conditions are favorable, the crop is quite vulnerable. If not addressed, serious crop losses can occur.
Agricultural crops are not the only plants that can be protected with the use of genetic engineering. In the first half of the twentieth century, the American chestnut, a major component of the eastern hardwood forest, was all but eliminated following the introduction of an Asian fungal disease, chestnut blight. Unlike Asian chestnut, American chestnut has absolutely no genetic resistance to the disease. Why is this important? American chestnut once made up 25% of the forest throughout much of its natural range. The leaves were a food source for many insects and the nutritious nuts provided food for animals including turkeys, bears, squirrels and more. And, of course, people enjoyed eating them, too. The wood was valued as a source of decay resistant lumber for construction and many other uses. Both traditional cross breeding and genetic engineering are possible solutions in the effort to bring back this significant species.
Farmers use many tools and techniques to prevent or manage plant pests and diseases. These include: Monitoring to detect pests early
Cultural practices such as crop rotation or trap crops
Resistant plant varieties (traditional breeding)
Appropriate fertilization and irrigation
Physical barriers and traps
Biological controls
Chemical controls (organic and conventional)
Genetically engineered crops
Genetic engineering may be used when other available tools are ineffective, unavailable, or when a clear benefit, such as reduced reliance on pesticides or increased yield, can be achieved.
Let’s look at three examples of traits used in agricultural crops today, what they do, how they work, which crops have them and why.
USDA revised regulations of GMO and gene edited plants. Here’s what it means. The long-awaited updates from the United States Department of Agriculture (USDA) to its genetically engineered (GE) organism regulation have been issued. This final rule completed a more than 10-year process started back in 2008 to revise regulations promulgated in 1987. This article discusses these new regulations and some of their potential impacts. Overall, the rule ignores concerns raised by some industry, consumer and environmental groups that the new regulations, including an option for developers to self-determine whether their products are regulated, and could lead to adverse environmental and/or agricultural impacts, potential food safety risks, trade disruptions and a lack of consumer acceptance of new food technologies.
USDA oversight from 1987 to the present
To understand the USDA’s new regulation of GE plants, it is important to know how the agency has regulated GE plants since 1987. USDA regulates the import, interstate movement, and environmental release of GE plants under its legal authority to manage “plant pests” under the Plant Protection Act. A “plant pest” is any organism “that can directly or indirectly injure, cause damage to, or cause disease in any plants or plant product.” Under USDA regulations, a GE plant has been considered a “potential” plant pest if any of its newly introduced DNA came from an organism on USDA’s list of plant pests, or if the method of introducing DNA into the plant’s genome involved an organism on USDA’s list of plant pests. For example, if a GE plant was developed using the plant pest Agrobacterium to introduce new DNA, as many are, it was regulated. However, if the same DNA were introduced using the gene-gun method of transformation, USDA would not regulate the GE plant.
Under those regulations (found at 7 CFR part 340), developers were required to submit their GE plant products to one of three oversight processes before environmental release.
The first process, known as “notification,” is used to regulate field trials of low-risk GE plants. The applicant provides the USDA with information detailing its trial and the agency has 30 days to decide whether to permit the trial to proceed. As many as 1,000 field trials are authorized yearly using this procedure.
The second process is “permitting,” which requires a more detailed application for any outdoor planting (e.g., field trial) of higher-risk GE plants. After reviewing the application, USDA may issue a permit authorizing the release. The USDA has issued hundreds of permits since 1987.
The third process involves a “petition for non-regulated status,” where a developer requests the USDA to determine—based on evidence from field trials—that the GE plant presents no plant pest risk and no longer requires regulation. The petition process is the primary path to commercialization and more than 140 plants have been deregulated.
For each regulatory process, the USDA is ensuring that the GE plant is not going to become a plant pest and cause harm to agricultural interests.
Up until 2011, every GE plant tested outdoors either submitted a notification or received a permit, and all commercialized plants satisfactorily completed the petition process. Then, in 2011, the USDA established a process whereby GE seed developers could ask the agency whether the GE plants they were developing required regulation, or whether they were exempt because they did not involve any plant pest components. The USDA responded to these “Am I regulated?” inquiries stating whether the GE plant was not regulated and could be planted without oversight. By the end of 2019, USDA determined that more than 85 plants did not fall within its regulatory authority and are exempt from oversight. So, over the last eight years, we have seen a decrease in how many GE plants USDA regulates.[1]
Revised regulations in 2020
The new rule (called the Sustainable, Ecological, Consistent, Uniform, Responsible, Efficient, (SECURE) Rule), which will be implemented over the next 18 months, applies to organisms produced through “genetic engineering,” which is defined to include “techniques that use recombinant, synthesized, or amplified nucleic acids to modify or create a genome.” This broad definition includes classical genetic engineering, which add one or more new genes to organism (transgenics, or what consumers consider GMOs), and newer gene editing techniques such as CRISPR, which can make edits within an organism’s existing genome.
While the definition captures all GE plants, the USDA exempts many of them from any oversight. First, it exempts products with a single sequence deletion, substitution, or addition (if the addition is from the plant’s gene pool). Second, it exempts any GE plant that has the same “plant-trait-mechanism of action” as any GE plant previous regulated by the USDA. This means that if the USDA previously regulated a GE plant, such as an glyphosate-tolerant variety of corn, a new GE glyphosate-tolerant corn is exempt if it employs the same mechanism of action (meaning it biologically operates the same way to provide tolerance). Developers can self-determine whether they qualify for these exemptions; confirmation of their self-determination from the USDA is not required and the agency need not be informed.
If a GE plant is not exempt, the developer can either: (1) apply for a permit if the GE plant has potential plant pest risks; or (2) seek a Regulatory Status Review (RSR). The RSR starts with an initial 180-day process where the USDA determines if the GE plant has any plausible plant pest risks. That initial RSR step is a closer look at the GE plant than the current “am I regulated?” process, but less detailed than the process used for “petitions for non-regulated status.” The USDA stated that the initial review does not require any plant-specific “laboratory or field-test data.” If the USDA decides there are no plausible risks, it sends a letter to the developer stating the plant is not regulated and publishes the letter on its website. If the USDA cannot conclude that there are no plausible risks, then the developer can either: (1) request that the USDA conduct the second part of the RSR, which is detailed evaluation of potential plant pest risks that can take up to 15 month); or (2) apply for a permit. The more lengthy and detailed RSR evaluation is comparable to the current “petition for non-regulated status” process and ends in the USDA determining either that the GE plant is not regulated or that it needs a permit. If a developer receives a permit from the USDA, any outdoor planting (e.g. a field trial or a commercial planting) is subject to restrictions to prevent inadvertent release into the environment and any adverse plant pest impacts. These are the same restrictions that virtually all GE plants were subject to prior to 2012 under the notification and permitting processes. Only GE plants that receive permits have any continued USDA oversight.
Worrisome aspects of SECURE
The exemptions are one worrisome aspect of the new SECURE rule. First, they are not supported by scientific evidence showing that these categories of GE plants do not pose risks. Instead, the USDA states that since a single deletion, substitution or addition produces a plant that could be achieved by conventional breeding methods, and because conventionally bred plants have not raised plant pest risks, gene-edited plants that are the same as products that could be achieved through conventional breeding will not pose plant pest risks. The problem with this argument is that a science-based regulatory system should base its oversight on whether the plant possesses traits that make it a potential plant risks, not the plant’s method of production. One reason the USDA revised its rules was to focus on the properties of a product, not how it was developed, yet that is the very approach these exemptions enshrine. While many, if not most, plants with a single deletion may not present any plant pest risks, if one does, shouldn’t USDA regulate it?
The second concern is that the developer self-determines if its product qualifies for an exemption. This sets up an inherent conflict of interest because developers have financial incentives to determine themselves exempt. While some developers will diligently determine the regulatory status of a GE plant, others may not. In addition, when a developer self-determines its product is exempt, neither the USDA nor the public know that the GE plant is being released into the environment and entering the food supply because there is no requirement to notify the agency of one’s self-determination. If the USDA does not know which GE plants are self-determined as exempt, how can it confirm that the determination is correct?
Positive aspects of SECURE
One positive of the new rule is the agency’s decision to limit the exemptions to single edits. The USDA reasons that while a single edit mimics a product that can be produced through conventional breeding methods, the same is not true for products with multiple edits. Therefore, if a developer makes two or more edits, the developer must apply for a permit or ask for an RSR. The first gene edited commercial product in the US—Calyxt’s high oleic soybean, which the USDA exempted under the “am I regulated?” process—would not be exempt under the new rules because it has two edited genes. If most gene-edited products end up having two or more edits, the exemptions may have limited applicability.
While multi-edited products are not automatically exempt, the USDA is likely to find in the initial step of the RSR process that many do not pose any plausible plant pest risk. So the result may be the same—these products are not regulated. However, at least the initial RSR determination (instead of a developer self-determination), is made public, so stakeholders will know which multi-edited products are entering the market.
Potential impacts
The USDA states that one goal of its revisions is to provide “regulatory relief,” and the final rule clearly achieves that. Many GE plants that historically required containment for field trials through either the notification or permitting process will no longer be subject to any substantive regulation. They either will be exempt or deemed to have no plausible plant pest risks through the initial step of the RSR process. What this means in practice is that GE plant developers (both private developers and academic scientists) can conduct field trials without any confinement conditions that ensure the GE plants do not persist in the environment after the trial is completed. The USDA stated in its proposed rule that it hopes developers voluntarily continue confinement measures, but that may or may not happen. GE plants have escaped from field trials with USDA oversight in the past and the likelihood of that happening will only increase without USDA oversight. That could mean new proteins inadvertently entering our food supply before they are deemed safe for human consumption. Experimental GE plants persisting in the environment after a field trial is concluded could also harm non-target organisms. Finally, if an unregulated GE plant escapes from a field trial and enters the export market, it could result in rejection of the US commodity because the experimental plant has not been approved in the importing country.
The final rule also fails to provide needed transparency on GE plants that will be commercialized. The USDA, food industry and consumers will be at the mercy of developers to make public information about products that they have deemed exempt. How will the food industry know which foods contain GE plants to ensure they are complying with export market legal requirements? How will food manufacturers and retailers answer questions from consumers asking whether their products contain ingredients from GE or gene-edited plants? If consumers are unable to access information about which GE plants are commercialized, will they become skeptical about those products and their safety? The lack of transparency inherent in the rule could result in international trade problems and misinformed consumers.
GE plants have provided benefits to farmers, the environment and consumers and are likely to continue to do so in the future. However, the USDA rule could impact the food industry’s acceptance of those products and fuel consumer suspicions about biotech crops and foods.
[1] While the USDA is the primary agency regulating GE plants, the FDA and EPA regulate subsets of GE plants. If a GE plant is used for food or feed, the FDA regulates it under a voluntary consultation process set up under the Federal Food, Drug and Cosmetic Act. If a GE plant produces a pesticide, the EPA regulates it as a plant-incorporated protectant under the Federal Insecticide, Fungicide, and Rodenticide Act.
-
 8:23:08
8:23:08
What If Everything You Were Taught Was A Lie?
11 days agoMystery Of Tartarian And Hidden Evidence To Lost History 3,000+ Images Of Forbidden History
3.27K2 -
 45:13
45:13
Standpoint with Gabe Groisman
2 hours agoEp. 61. Antisemitism Across America & Fighting Harvard. Shabbos Kestenbaum
513 -
 50:16
50:16
Graham Allen
3 hours agoWelcome To The “Non-Woke” Christmas Episode! You Won’t Believe What Happens!!
14.2K32 -
 LIVE
LIVE
Matt Kohrs
11 hours agoNew Market Highs Incoming!!! || The MK Show
1,262 watching -
 37:13
37:13
BonginoReport
4 hours agoOpenAI Whistleblower Commits "Suicide" (Ep.105) - 12/16/2024
24.5K48 -
 LIVE
LIVE
Wendy Bell Radio
5 hours agoABC News Can Suck It
13,484 watching -
 LIVE
LIVE
Jeff Ahern
2 hours agoMonday Madness with Jeff Ahern ( the truth is out there)
432 watching -
 1:27:08
1:27:08
Game On!
14 hours ago $5.63 earnedTravis Hunter wins Heisman, Mahomes goes DOWN, and so much more from the sports weekend!
82.3K3 -
![Attack of the DRONES! [D/REZZED Paranormal News]](https://1a-1791.com/video/s8/1/0/_/B/L/0_BLv.0kob-small-Attack-of-the-DRONES-DREZZE.jpg) 31:19
31:19
Clownfish TV
20 hours agoAttack of the DRONES! [D/REZZED Paranormal News]
25.1K12 -
 21:12
21:12
Degenerate Jay
23 hours ago $2.81 earnedIs Marvel Rivals Worth Playing?
47.1K4