Premium Only Content
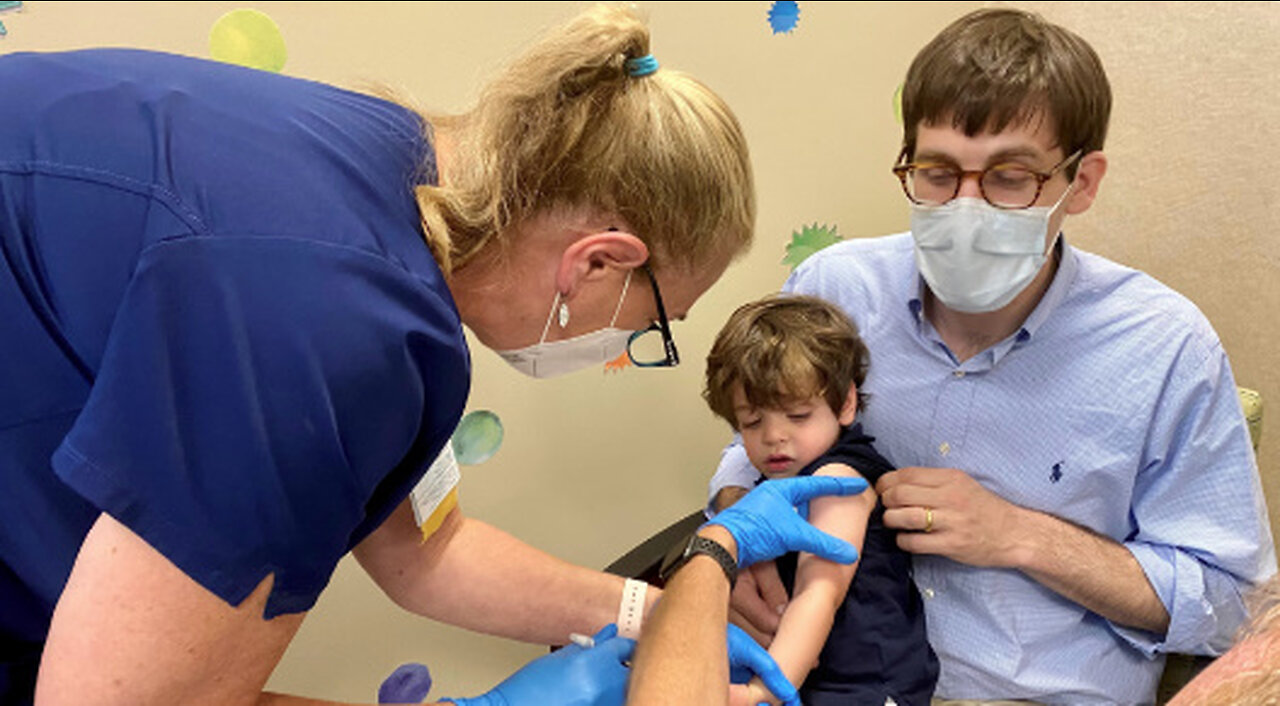
Covid Vaccine Clinical Trial for Children Ages 6 Months to 5 Years
CHARLOTTE, N.C., May 9, 2022 – Atrium Health Levine Children’s is the first and only site in the Charlotte area to offer the Pfizer-BioNTech COVID-19 vaccine study to healthy children ages six months old to under five years of age. The study will evaluate safety, tolerability and immune response to the vaccine among this age group. All participating children will be closely monitored by Levine Children’s highly-skilled clinical team as part of the study. Any changes to national recommendations for future Emergency Use Authorization (EUA) approvals will be incorporated into the study.
“The Pfizer-BioNTech vaccine has been shown to be very safe and effective in adults, adolescents and school-age children,” said Dr. Christine Turley, vice chair for research at Levine Children’s and principal investigator for the Charlotte trial. “We’re building on the strong safety profile that exists for adults and older children. We will be studying how well a lower dose works for young children in providing the level of protection they need. We have every reason to believe this study will show the vaccine to be a strong option to offer children protection against the COVID-19 virus.”
The vaccine being studied is the same one that has been authorized for people ages five and older, adjusted to a lower dose. Additionally, a third dose is being tested in this age group, to ensure the immune protection reaches the level that has been beneficial in older children. In adults, this vaccine has demonstrated very high protection against the COVID-19 virus and serious disease.
“Over 350 million doses of Pfizer’s COVID-19 vaccine have been given in the U.S. since the vaccine has been emergency authorized and now fully approved,” Turley said. “This gives us an enormous amount of safety information in a very rapid timeframe, because it allows us to quickly learn about rare side effects. That gives me a lot of confidence from a safety standpoint.”
“Since the beginning of the COVID-19 pandemic, a lot of people have been living with a great deal of worry,” Turley said. “Parents have been waiting very anxiously because there is currently no way to protect our youngest children. This vaccine study is a hopeful one and gives families and children the opportunity to be protected while becoming a part of history.”
With more than 20 years of personal experience, Turley is known for her work in vaccine development, advocacy and education. The wealth of experience between Turley and the research team at Levine Children’s, as well as the previous high-quality vaccine studies performed by the team, are key reasons the health system was chosen to lead this specific research in Charlotte. Atrium Health is the only health system in the region to host COVID-19 vaccine trials down to the infancy age group.
The study is taking place around the country, and local enrollment is expected to take place quickly as there is an urgent demand for a safe, effective vaccine for this age group. Families choosing to enroll their children will be part of the pivotal three-dose, low-dose vaccine study.
-
 1:51
1:51
VikingVigilante
1 month agoTulsi Gabbard: The US funds 300 biolabs around the world.
3741 -
 LIVE
LIVE
Donald Trump Jr.
4 hours agoHappy Festivus: Airing Our Grievances and Stopping The Swamp w/Sean Davis | TRIGGERED Ep.201
4,212 watching -
 LIVE
LIVE
The Jimmy Dore Show
53 minutes agoMedia ADMITS They Lied About Biden’s Decline! SNL Audience CHEERS For Luigi Mangione!
7,704 watching -
 LIVE
LIVE
Robert Gouveia
2 hours agoMatt Gaetz REJECTS Report, Sues Committee; Luigi Fan Club Arrives; Biden Commutes; Festivus Waste
2,495 watching -
 58:10
58:10
Kimberly Guilfoyle
4 hours agoAmerica is Back & The Future is Bright: A Year in Review | Ep. 183
25.7K16 -
 3:03:27
3:03:27
vivafrei
9 hours agoEp. 242: Barnes is BACK AGAIN! Trump, Fani, J6, RFK, Chip Roy, USS Liberty AND MORE! Viva & Barnes
71.9K21 -
 LIVE
LIVE
Dr Disrespect
7 hours ago🔴LIVE - DR DISRESPECT - MARVEL RIVALS - GOLD VANGUARD
3,493 watching -
 1:15:00
1:15:00
Awaken With JP
6 hours agoMerry Christmas NOT Happy Holidays! Special - LIES Ep 71
84.9K87 -
 1:42:21
1:42:21
The Quartering
7 hours agoTrump To INVADE Mexico, Take Back Panama Canal Too! NYC Human Torch & Matt Gaetz Report Drops!
78.9K45 -
 2:23:15
2:23:15
Nerdrotic
7 hours ago $9.32 earnedA Very Merry Christmas | FNT Square Up - Nerdrotic Nooner 453
57.3K4