Premium Only Content
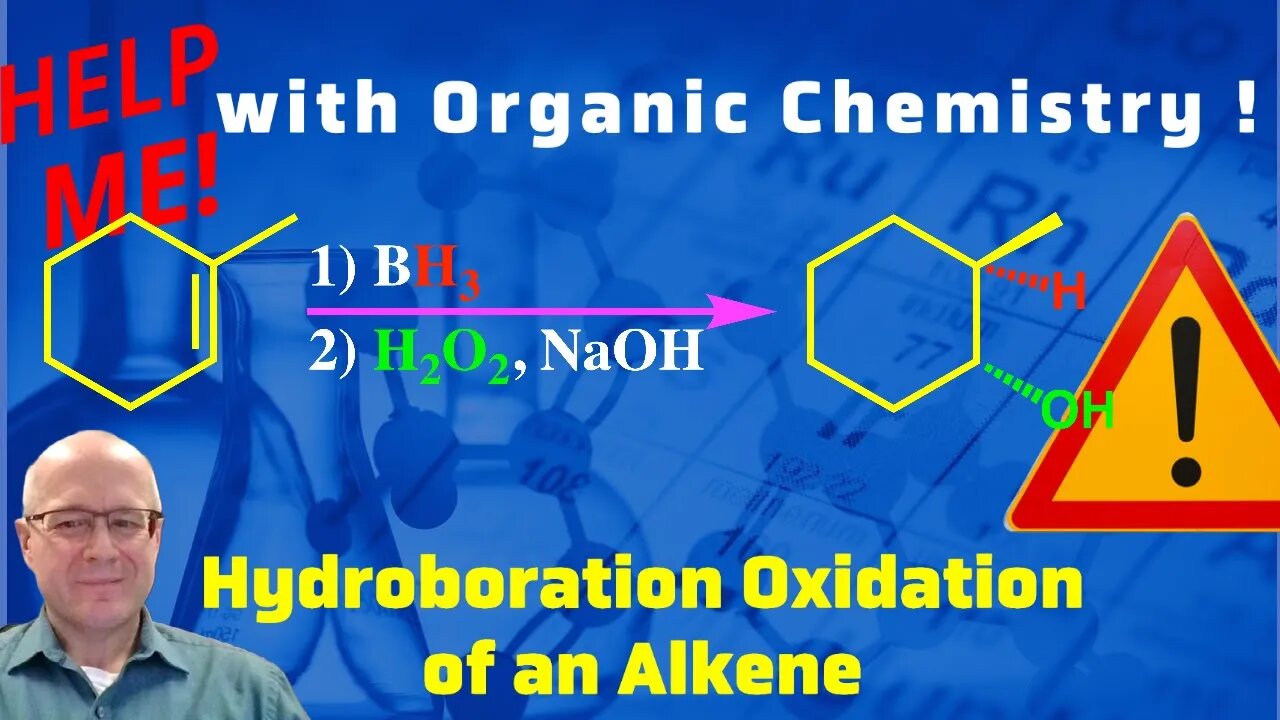
Hydroboration Oxidation Reaction of Alkenes to Form Alcohols Help Me With Organic Chemistry!
The E2 reaction delivers alkene products. A reaction that follows an E2 mechanism becomes interesting when there are stereocenters that can deliver two diastereomers known as E or Z (cis or trans. The outcome of an E2 reaction is entirely predictable when you follow the rules. In this video I will show you how to use Newman projections to predict the E or Z cis or trans out come of these reactions. I will also show you how to do this without using a Newman Projection and get the same result!
The E2 mechanism is second order. This means that the base and the substrate exist together in the transition state of the rate determining step.
Failing organic chemistry? You do not have to fail Organic Chemistry!
This video is part of a series called How to be Successful in Organic Chemistry. In this series I go over numerous problems that a student could expect to see in there organic chemistry 1 course. Doing organic chemistry practice problems will make you more successful in organic chemistry and biochemistry.
I recommend that you download the problem from the link below and attempt the problem yourself and use this video to correct your work.
Download the problem from this video at the following link:
Good Luck and Good Chemistry!
Please subscribe to my channel by clicking the link below!
https://www.youtube.com/c/AllInwithDrBetts?sub_conformation=1
Like this video and leave a comment below!
-
 3:27
3:27
Chemistry Tutor
1 year agoConverting Grain Measurements to Milliliters of Morphine: A Comprehensive Guide
93 -
 2:12:41
2:12:41
TheSaltyCracker
8 hours agoMSM Implodes After Trump Win ReeEEeE Stream 11-17-24
179K369 -
 4:31
4:31
SLS - Street League Skateboarding
10 days agoFuna Nakayama 3rd Place at SLS Sydney 2024 | Best Tricks
55.5K7 -
 2:15:24
2:15:24
vivafrei
16 hours agoEp. 236: BARNES IS BACK! Election Recap! Trump Nominees! Trump Persecutions - Wha's Next? & MORE!
203K204 -
 6:25:47
6:25:47
SynthTrax & DJ Cheezus Livestreams
15 hours agoDJ Cheezus & DEF JAM Fight for NY on PS2 - Hip Hop Violence and Vibes (1pm PST / 4pm EST)
82.2K4 -
 2:01:47
2:01:47
Nerdrotic
11 hours ago $13.16 earnedEgypt, Peru and Guatemala Luke Caverns RETURNS! | Forbidden Frontier #082
67.7K10 -
 LIVE
LIVE
Vigilant News Network
11 hours agoFDA Approves Trials for New “Pandemic” Vaccine | Media Blackout
2,939 watching -
 4:06:21
4:06:21
GamerGril
11 hours agoIM THE GREASTEST OF ALL TIME AT.... CHAOS | DAYS GONE
114K12 -
 3:00:18
3:00:18
Due Dissidence
1 day agoTHE PEOPLE VS NATURE by Kevin Augustine - plus a talkback w/ Jimmy Dore
130K11 -
 6:02:06
6:02:06
Rotella Games
17 hours agoMake the Hood Great Again | Day 3 | GTA San Andreas
85.7K5