Premium Only Content
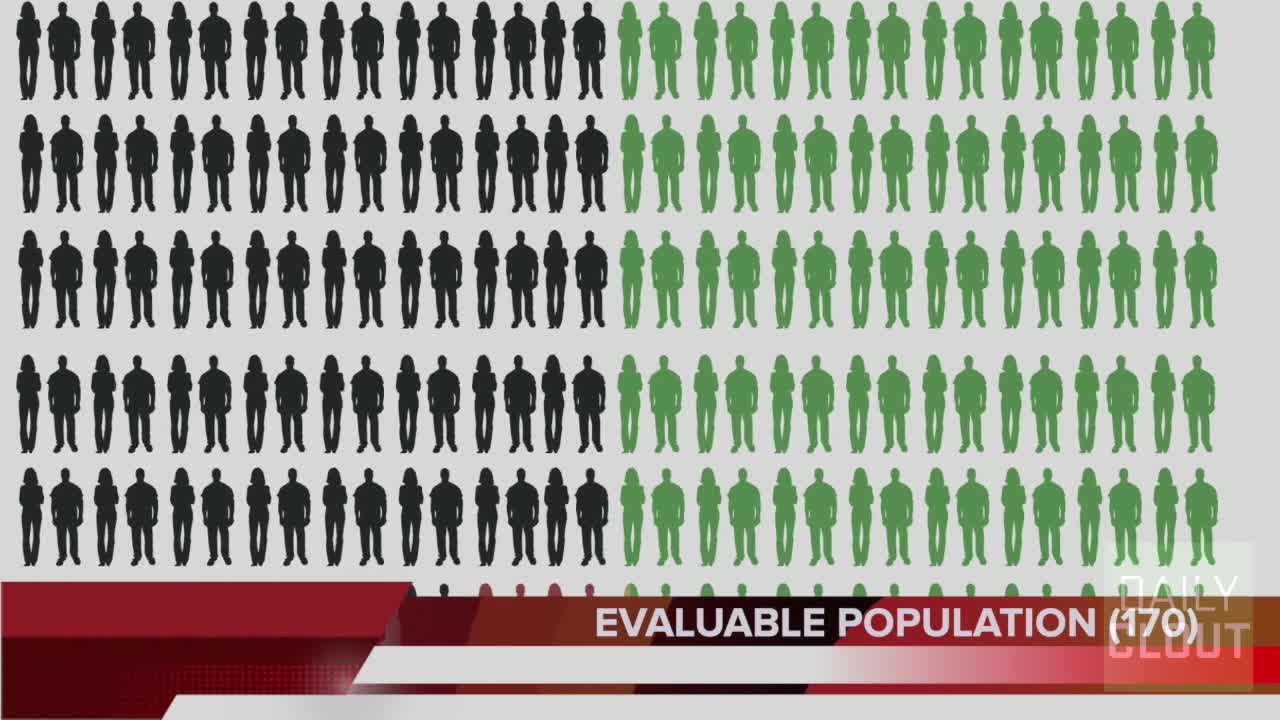
How Pfizer's EUA Was Granted Based on Less Than 0.4% of Clinical Trial Participants
1. The pivotal Pfizer trial upon which the Emergency Use Authorization (EUA) for the COVID -19 vaccines was on the endpoint of efficacy of the vaccines.
2. This was to be done when the number of eligible patients ("evaluable efficacy population") reached a threshold for analysis. The threshold was 164 trial participants.
3. We show that this threshold was likely not met and that the granting of the EUA should revisited.
4. There seemed to be a deliberate ambiguity left in the trial protocol documents to allow patients who would otherwise be deemed to have violated the trial protocol to still be included in the analysis. There was marked difference in what was implicitly stated to be the appropriate dosing interval between Dose 1 and Dose 2 in the trial protocol and all subsequent 14 protocol amendments, and what was accepted and approved in the EUA memorandum in December 2020.
5. We also show that patients in the final efficacy analysis had other significant protocol deviations, besides just going outside of the protocol's dosing interval.
-
 5:35
5:35
Cooking with Gruel
21 hours agoMaking Fresh Salted Caramel
22.6K5 -
 16:16
16:16
DeVory Darkins
18 hours ago $6.76 earnedMedia Panics after Trump Threatens to Sue Media for Defamation
34.3K64 -
 2:15:34
2:15:34
Matt Kohrs
5 hours agoFed Powell Speech & FOMC Rate Decision
23.3K2 -
 LIVE
LIVE
StoneMountain64
4 hours agoThe MOST hyped game of the YEAR
218 watching -
 1:50:12
1:50:12
The Quartering
19 hours agoTim Pool SELLS TO DAILY WIRE? Never Eat Hot Dogs Again, Drones & More
85.8K24 -
 1:17:48
1:17:48
Tucker Carlson
5 hours agoTom Homan’s Plan to Destroy the Cartel Empire, End Child Trafficking, and Secure the Border for Good
129K156 -
 1:06:28
1:06:28
Russell Brand
6 hours agoWho Ordered the Hit on Russia’s General Krylov? - SF516
143K257 -
 54:50
54:50
The Kevin Trudeau Show
6 hours agoWhat Men Do Wrong In Relationships | Ep. 75
16.5K7 -
 12:41
12:41
Gun Owners Of America
5 hours agoWe're Fighting Back Against Mexico In Court!
22.9K5 -
 2:57:11
2:57:11
The Charlie Kirk Show
5 hours agoThe CR Quagmire + An Hour of PBD + Catholic in Hollywood | Davis, Patrick Bet David, Rep. Burlison
129K34