Premium Only Content
Introducing VAERS Explorer
What is VAERS EXPLORER, and what is it for?
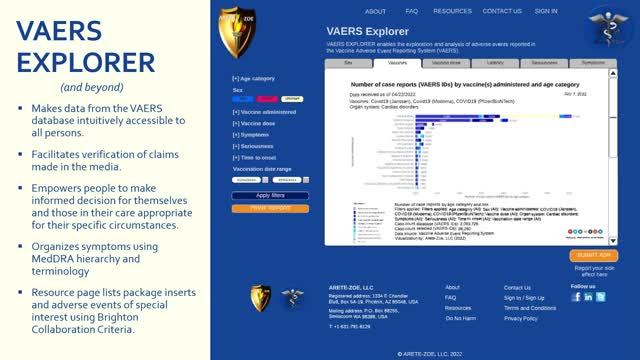
VAERS EXPLORER is a web application and a research tool intended to enable users to explore and analyze vaccine adverse events reported in the Vaccine Adverse Event Reporting System (VAERS) to create customized reports via filter selection directly from our website.
VAERS EXPLORER will empower the public to find relevant, accurate, and reliable information on vaccine safety to better participate in health care for themselves and those in their care. Additionally, VAERS Explorer supports analytical inquiry by healthcare professionals and researchers concerned with vaccine safety. Website visitors can organize queries and download relevant information with customized graphs. They can learn about both known side effects as declared in approved product labeling and actual adverse events as reported.
For healthcare practitioners, VAERS EXPLORER will offer a detailed analysis of symptoms and clinical syndromes of cases reported in VAERS. Real-time insight into reported cases will facilitate the detection of potential side effects in their practice and their appropriate follow-up.
VAERS EXPLORER can also serve as a comprehensive research tool that generates reports for regulatory professionals and researchers.
VAERS EXPLORER currently consists of a backend database of VAERS data that has data elements correlated to facilitate meaningful analysis and usability.
What is VAERS?
The Vaccine Adverse Event Reporting System (VAERS) is a U.S. national early warning system designed to detect vaccine safety issues. VAERS contains reports of adverse events from patients, healthcare professionals, and vaccine manufacturers. Manufacturers must report any side effects that come to their attention. Healthcare providers must report adverse events listed in the VAERS Table of Reportable Events Following Vaccination that occur within a specified timeframe following vaccination. The number of reports alone does not imply a causal association between a vaccine and an adverse event. The interpretation of VAERS data requires careful evaluation in the context of other scientific knowledge.
How can you currently access data in VAERS?
VAERS Data is available to the public via the web application CDC Wonder. However, CDC Wonder is not intuitive, and getting a meaningful output is difficult even for a safety professional. Knowledge of the data structure and specialized terminology is essential to interact with the data successfully.
What makes VAERS EXPLORER different?
VAERS EXPLORER is designed as a one-stop shop for vaccine safety.
VAERS EXPLORER makes VAERS data widely accessible and understandable by refining the data through selectable filters and producing visualizations with accompanying information tables to facilitate meaningful analysis and early indication of possible adverse events.
VAERS EXPLORER provides additional value by providing essential resources to better judge own risk based on individual circumstances. As a result, we empower people to make informed decisions to control their healthcare better.
VAERS EXPLORER enables patients to better engage their healthcare providers. Reports generated by VAERS EXPLORER provide comprehensive information about vaccine safety, along with data on existing case reports of adverse events and the implications/ significance in the context of their specific health profile.
VAERS EXPLORER facilitates analysis of symptoms by organizing the data using MedDRA system organ class hierarchy, allowing intuitive orientation in most likely areas of concern.
VAERS EXPLORER enables analysis of clinical syndromes. A combination of side effects experienced can amount to a specific diagnosis that would not be otherwise detectable.
Who are we?
Veronika Valdova is a veterinarian working as a consultant in the safety of drugs and medical devices. She knows the regulatory requirements and industry practices required to ensure that marketed products' benefit: risk profile remains favorable. She is also the principal architect of VAERS EXPLORER.
Ronald L Sheckler is a multi-disciplined consultant in Business Intelligence and operational risk management who retired from the U.S. Army after 31 years in Special Forces. He is an experienced and successful developer of full spectrum multi-service and inter-agency operational plans and policy. His contributions to VAERS EXPLORER are refining the concept, design, implementation, and business model.
The primary business of our consultancy ARETE-ZOE, LLC is the safety of medical devices and pharmaceutical products.
GoFundMe: https://www.gofundme.com/f/build-vaers-explorer
Project description: https://www.aretezoe.com/vaers-explorer
Facebook: https://www.facebook.com/VAERSExplorer/
-
 9:49
9:49
Tundra Tactical
8 hours ago $13.14 earnedThe Best Tundra Clips from 2024 Part 1.
76.9K7 -
 1:05:19
1:05:19
Sarah Westall
9 hours agoDying to Be Thin: Ozempic & Obesity, Shedding Massive Weight Safely Using GLP-1 Receptors, Dr. Kazer
70.5K19 -
 54:38
54:38
LFA TV
1 day agoThe Resistance Is Gone | Trumpet Daily 12.26.24 7PM EST
51.7K9 -
 58:14
58:14
theDaily302
17 hours agoThe Daily 302- Tim Ballard
52.8K6 -
 13:22
13:22
Stephen Gardner
11 hours ago🔥You'll NEVER Believe what Trump wants NOW!!
102K237 -
 54:56
54:56
Digital Social Hour
1 day ago $10.51 earnedDOGE, Deep State, Drones & Charlie Kirk | Donald Trump Jr.
56.4K5 -
 DVR
DVR
The Trish Regan Show
13 hours agoTrump‘s FCC Targets Disney CEO Bob Iger Over ABC News Alleged Misconduct
61.7K37 -
 1:48:19
1:48:19
The Quartering
13 hours agoElon Calls White People Dumb, Vivek Calls American's Lazy & Why Modern Christmas Movies Suck!
143K112 -
 2:08:42
2:08:42
The Dilley Show
14 hours ago $36.74 earnedH1B Visa Debate, Culture and More! w/Author Brenden Dilley 12/26/2024
123K42 -
 4:55:59
4:55:59
LumpyPotatoX2
16 hours agoThirsty Thursday on BOX Day - #RumbleGaming
113K7