Premium Only Content
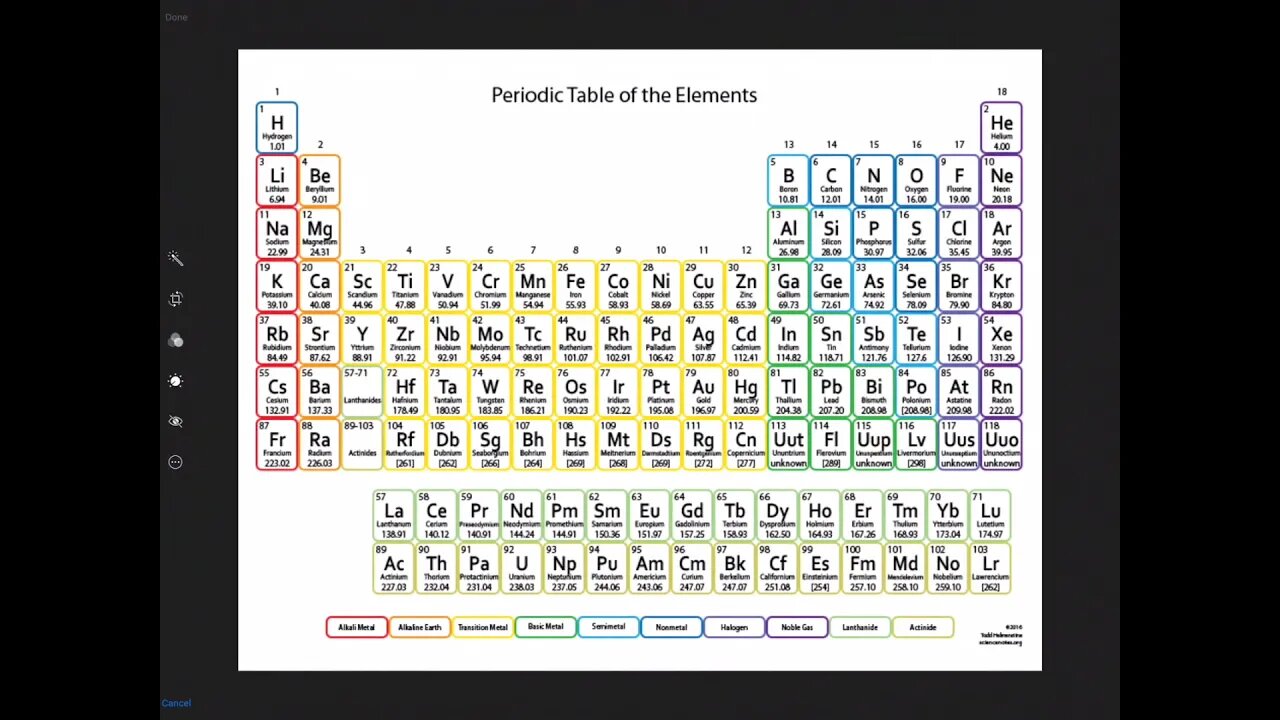
Calculating molarity of a solution chemistry problem solving made easy!
This video will demonstrate to the nursing student how to calculate molarity. Molarity is the relationship between the moles of a solute and the liters of a solution. Calculating molarity is as simple as dividing the moles of a solute by the liters of solution.
The molarity formula is Molarity = grams or solute / liters of solution. The real complication for this type of problem is calculating moles of solute. However, you should not let that intimidate you. The moles of solute can be easily calculated by simply dividing the grams of solute by the molar mass of the solute. Of course, molarity problems can be further complicated by forcing the student to also calculate the molar mass of the solute from the molecular formula. Do not get discouraged! Molar mass can be easily calculated by adding up the molar masses of each element in the formula.
For more on calculating molar mass please see the following video:
Molarity is an important concentration unit used in chemistry. The better you understand molarity the easier a lot of other chemistry problems will be for you!
Please like this video and leave a comment below! It really helps and is so encouraging.
Please subscribe to my channel by clicking the link below!
https://www.youtube.com/c/AllInwithDrBetts?sub_conformation=1
Leave a like and comment below!
#molarity
#molarmass
#calculationofmoles
#molarityequation
#moles
#solute
#solution
#nursingchemistry
#nursingchem
-
 1:09:09
1:09:09
Chemistry Tutor
1 year ago $0.03 earnedAll About the SN1 Mechanism - Lecture 29 has need for more editing
76 -
 1:03:51
1:03:51
Flyover Conservatives
1 day agoGeneration Z’s Revolution: 17 Year Old Author on the Return of Faith, Family, and the End of Feminism - Hannah Faulkner; Economic Update - Dr. Kirk Elliott | FOC Show
32.2K2 -
 1:12:43
1:12:43
Adam Does Movies
10 hours ago $16.89 earnedMoviegoers Are Singing Now! + Lilo & Stitch + Sonic 3 - LIVE!
76.7K7 -
 1:26:05
1:26:05
Donald Trump Jr.
13 hours agoRegime Media Imploding: What’s Next for MSNBC? Plus Michael Knowles & Alex Marlow | TRIGGERED Ep.194
220K206 -
 37:26
37:26
Glenn Greenwald
10 hours agoGlenn Takes Your Questions: On Trump's Cabinet, The G20 Summit, and More | SYSTEM UPDATE LOCALS SPECIAL
85.5K52 -
 2:10:20
2:10:20
We Like Shooting
17 hours ago $2.14 earnedWe Like Shooting 586 (Gun Podcast)
21K1 -
 52:14
52:14
Uncommon Sense In Current Times
12 hours ago $0.65 earned“Pumpkin Pie Politics: Bridging the Thanksgiving Divide to Protect The Family"
15.7K1 -
 1:01:28
1:01:28
The StoneZONE with Roger Stone
7 hours agoWhy Jack Smith Owes Americans Millions of Dollars for Fake Investigations | The StoneZONE
36.5K6 -
 3:50:40
3:50:40
Tundra Gaming Live
11 hours ago $2.61 earnedThe Worlds Okayest War Thunder Stream
37.4K -
 2:22:30
2:22:30
WeAreChange
9 hours agoBREAKING: Biden Admin Could SEND NUKES To Ukraine?! UK & France To Send Troops? w/ Roger Stone
52.3K18