Premium Only Content
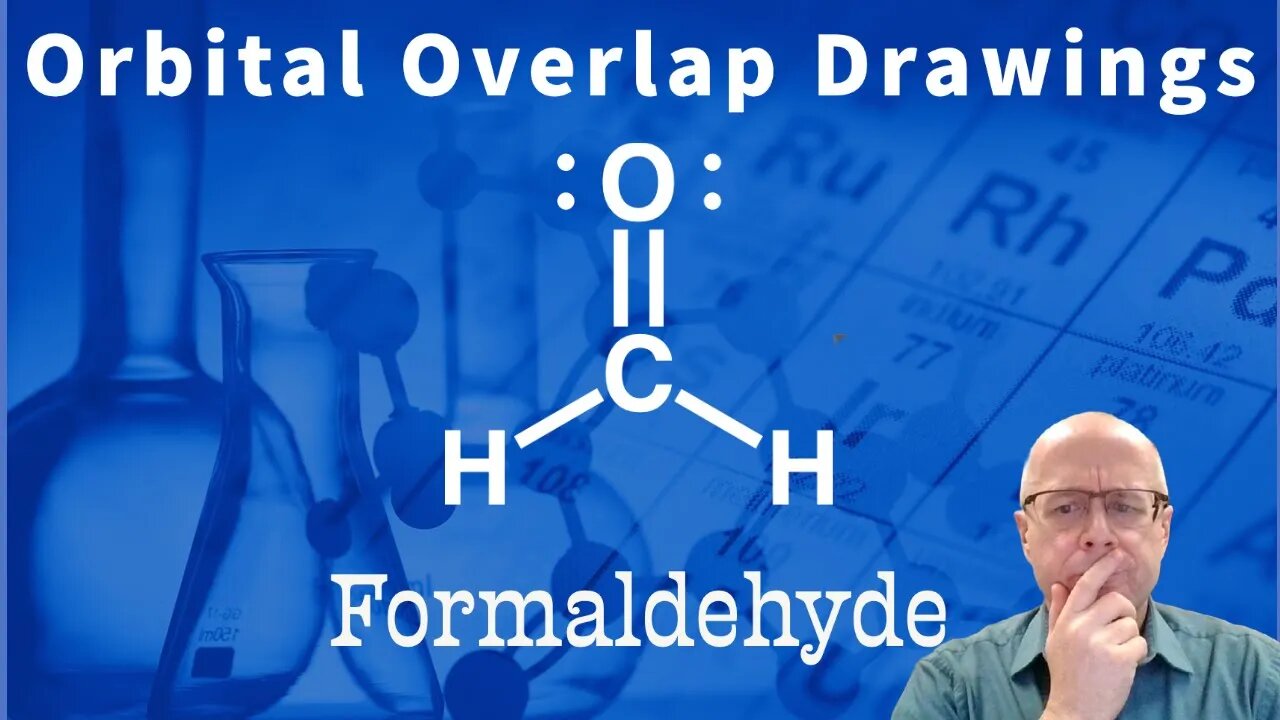
Organic Chemistry Orbital Overlap Problem: Formaldehyde (carbonyl) sp2
Being able to visualize the bonding orbitals of an organic molecule is important to your success in organic chemistry. Indeed, understanding orbital bonding and hybrid orbitals will help you to understand reactivity as your organic chemistry class moves forward into more difficult subjects. Sp3, sp2 and sp orbitals are involved in sigma bonding and p orbitals are involved in pii bonding.
In this video I am drawing the orbital overlap drawing of formaldehyde. In formaldehyde the carbon is sp2 hybridized. This means it has three (3) sp2 orbitals and one (1) unhybridized p orbital. The sp2 orbitals make the sigma bonds and the p orbital makes the pi bond. The oxygen atom will have three (3) sp2 orbitals and one (1) unhybridized p orbital. One of the sp2 orbitals will for a sigma bond with carbon and the other two (2) sp2 orbitals will hold one lone pair each. The p orbital of oxygen will form a pi bond with carbon.
It is important to understand the difference between sigma and pi bonds. It is also important to understand the sigma bond orbitals and the pi bond orbitals.
I recommend that you download the problem from the link below and attempt the problem yourself and use this video to correct your work.
Download the problem from this video at the following link:
https://www.dropbox.com/s/i5iig4kxim9zuhd/formaldehyde%20hybridized%20orbitals.pdf?dl=0
Good Luck and Good Chemistry!
Please subscribe to my channel by clicking the link below!
https://www.youtube.com/c/AllInwithDrBetts?sub_conformation=1
Like this video and leave a comment below!
#orbitals #sp2 #formaldehyde
#organicchemistry
#organiccompounds
#organicproblems
#organictutor
#hybridorbitals
#hybrid
00:00 Introduction
00:16 Orbitals of formaldehyde
02:25 conclusion
-
 6:07
6:07
Chemistry Tutor
1 year agoHow to Draw a Lewis Structure From a Molecular Formula C5H8Cl2 Help with Chemistry!
77 -
 1:42:21
1:42:21
The Quartering
17 hours agoTrump To INVADE Mexico, Take Back Panama Canal Too! NYC Human Torch & Matt Gaetz Report Drops!
166K110 -
 2:23:15
2:23:15
Nerdrotic
16 hours ago $13.78 earnedA Very Merry Christmas | FNT Square Up - Nerdrotic Nooner 453
125K12 -
 1:14:05
1:14:05
Tucker Carlson
16 hours ago“I’ll Win With or Without You,” Teamsters Union President Reveals Kamala Harris’s Famous Last Words
224K377 -
 1:58:31
1:58:31
The Dilley Show
16 hours ago $36.14 earnedTrump Conquering Western Hemisphere? w/Author Brenden Dilley 12/23/2024
166K50 -
 1:09:59
1:09:59
Geeks + Gamers
17 hours agoSonic 3 DESTROYS Mufasa And Disney, Naughty Dog Actress SLAMS Gamers Over Intergalactic
113K21 -
 51:59
51:59
The Dan Bongino Show
18 hours agoDemocrat Donor Admits The Scary Truth (Ep. 2393) - 12/23/2024
931K3.11K -
 2:32:15
2:32:15
Matt Kohrs
1 day agoRumble CEO Chris Pavlovski Talks $775M Tether Partnership || The MK Show
143K36 -
 28:23
28:23
Dave Portnoy
1 day agoDavey Day Trader Presented by Kraken - December 23, 2024
173K44 -
 59:29
59:29
BonginoReport
20 hours agoTrump, Murder Plots, and the Christmas Miracle: Evita + Jack Posobiec (Ep.110) - 12/23/2024
175K152