Premium Only Content
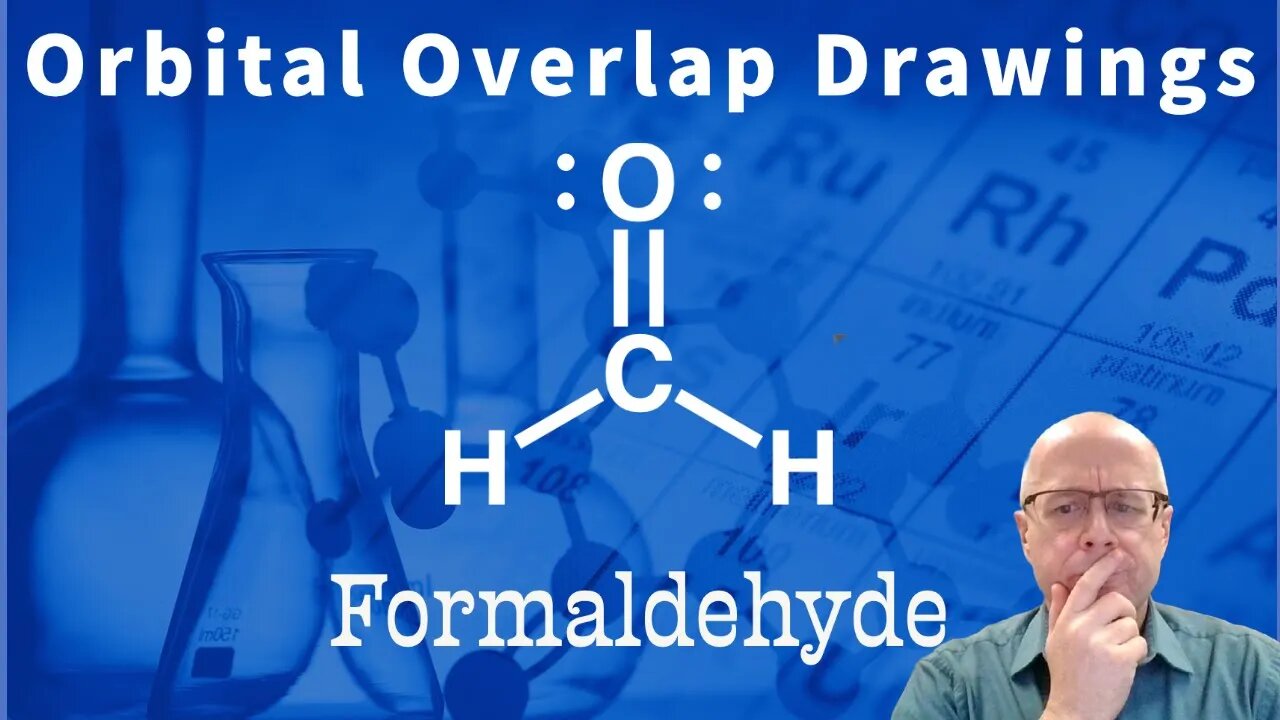
Organic Chemistry Orbital Overlap Problem: Formaldehyde (carbonyl) sp2
Being able to visualize the bonding orbitals of an organic molecule is important to your success in organic chemistry. Indeed, understanding orbital bonding and hybrid orbitals will help you to understand reactivity as your organic chemistry class moves forward into more difficult subjects. Sp3, sp2 and sp orbitals are involved in sigma bonding and p orbitals are involved in pii bonding.
In this video I am drawing the orbital overlap drawing of formaldehyde. In formaldehyde the carbon is sp2 hybridized. This means it has three (3) sp2 orbitals and one (1) unhybridized p orbital. The sp2 orbitals make the sigma bonds and the p orbital makes the pi bond. The oxygen atom will have three (3) sp2 orbitals and one (1) unhybridized p orbital. One of the sp2 orbitals will for a sigma bond with carbon and the other two (2) sp2 orbitals will hold one lone pair each. The p orbital of oxygen will form a pi bond with carbon.
It is important to understand the difference between sigma and pi bonds. It is also important to understand the sigma bond orbitals and the pi bond orbitals.
I recommend that you download the problem from the link below and attempt the problem yourself and use this video to correct your work.
Download the problem from this video at the following link:
https://www.dropbox.com/s/i5iig4kxim9zuhd/formaldehyde%20hybridized%20orbitals.pdf?dl=0
Good Luck and Good Chemistry!
Please subscribe to my channel by clicking the link below!
https://www.youtube.com/c/AllInwithDrBetts?sub_conformation=1
Like this video and leave a comment below!
#orbitals #sp2 #formaldehyde
#organicchemistry
#organiccompounds
#organicproblems
#organictutor
#hybridorbitals
#hybrid
00:00 Introduction
00:16 Orbitals of formaldehyde
02:25 conclusion
-
 3:21
3:21
Chemistry Tutor
1 year ago $0.04 earnedNursing School Math Problems: Milliters of Amoxicillin per Day to Total Milligrams of Amoxicillin
68 -
 LIVE
LIVE
GamersErr0r
16 hours agoOverwatch 2
258 watching -
 LIVE
LIVE
Phyxicx
3 hours agoRocket League with Friends! - 11/22/2024
111 watching -
 LIVE
LIVE
STARM1X16
3 hours agoFriday Night Fortnite
107 watching -
 29:51
29:51
Afshin Rattansi's Going Underground
18 hours agoJimmy Dore on Ukraine & WW3: Biden Wants a War that Trump CAN’T Stop, ONLY Hope is Putin’s Restraint
56.7K17 -
 DVR
DVR
Fresh and Fit
5 hours agoExposing WHO Killed JFK w/ Cory Hughes & Tommy Sotomayor
72.6K23 -
 LIVE
LIVE
RanchGirlPlays
5 hours ago🔴 Red Dead Redemption: Let's go help De Santa 🤠
226 watching -
 LIVE
LIVE
Man in America
11 hours agoWHAT?! Trump & the Fed are DISMANTLING the Global Banking Cartel!? w/ Tom Luongo
2,115 watching -
 LIVE
LIVE
HELMET FIRE
1 hour agoDEADROP IS BACK!
118 watching -
 LIVE
LIVE
I_Came_With_Fire_Podcast
6 hours agoLive Fire (No Exercise) with DAN NUNN!!!
239 watching