Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
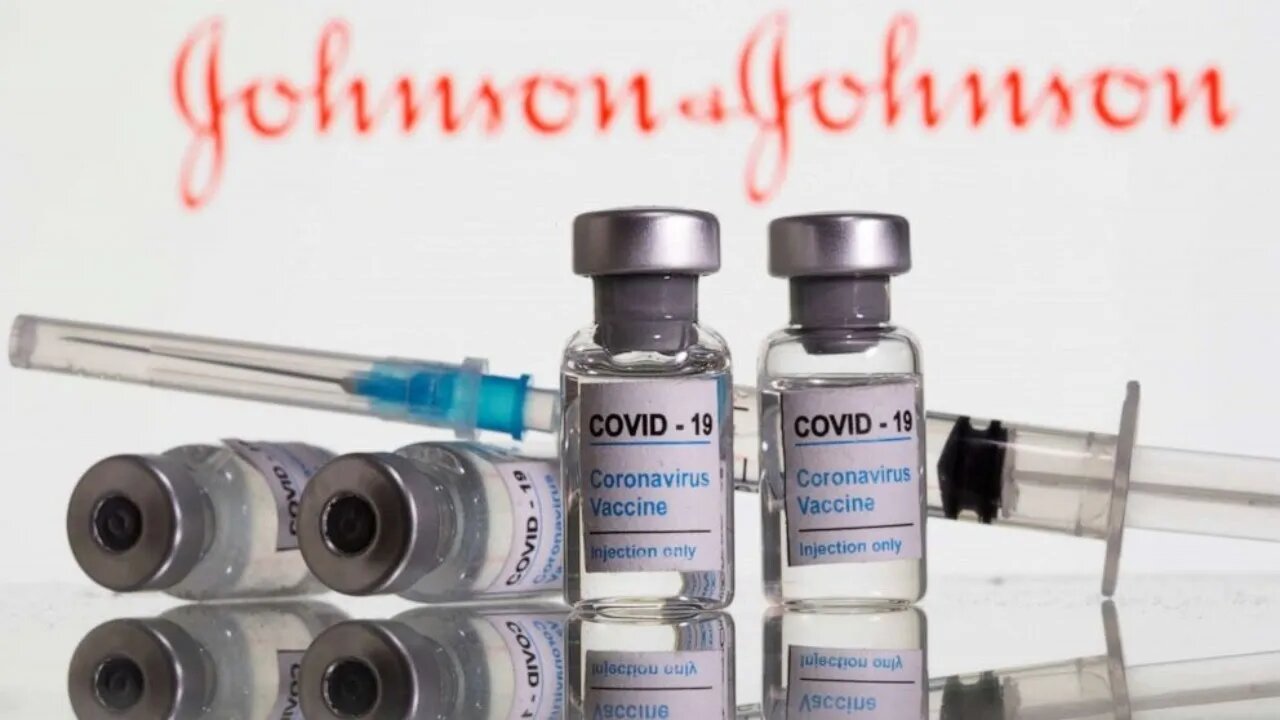
FDA to discuss rare side effects type of blood clot from J&J vaccine
3 years ago
15
The United States recommends a "discontinuation" of single-dose in the administration of the Johnson & Johnson COVID-19 vaccine to investigate reports of potentially dangerous blood clots.
In a joint statement Tuesday, the Centers for Disease Control and Prevention and the Food and Drug Administration said they were investigating unusual clots in six women that occurred 6 to 13 days after vaccination. Clots occur in the veins that drain blood from the brain and occur with low platelets. The six cases were women between the ages of 18 and 48.
-~-~~-~~~-~~-~-
Please watch: "Reina Rose interview Phil Cooke Hollywood Christian Producer"
https://www.youtube.com/watch?v=ogBS-do5g9k
-~-~~-~~~-~~-~-
Loading comments...
-
 47:50
47:50
Candace Show Podcast
6 hours agoBREAKING: Judge Makes Statement Regarding Taylor Swift's Text Messages. | Candace Ep 155
94.3K104 -
 DVR
DVR
Josh Pate's College Football Show
2 hours agoCFB’s Most Hated Teams | FSU & Clemson Future | Big Ten Win Totals | Star Rankings Overrated?
5.76K -
 1:33:47
1:33:47
CatfishedOnline
4 hours agoGoing Live With Robert - Weekly Recap
19.4K -
 55:18
55:18
LFA TV
1 day agoEurope’s Sudden Turn Against America | TRUMPET DAILY 3.6.25 7PM
25.5K3 -
 4:21
4:21
Tundra Tactical
4 hours ago $1.38 earnedPam Bondi MUST Enforce Due Process NOW!
18.4K1 -
 56:42
56:42
VSiNLive
5 hours agoFollow the Money with Mitch Moss & Pauly Howard | Hour 1
43.6K1 -
 1:05:32
1:05:32
In The Litter Box w/ Jewels & Catturd
1 day agoShalom Hamas | In the Litter Box w/ Jewels & Catturd – Ep. 756 – 3/6/2025
98.7K37 -
 1:23:00
1:23:00
Sean Unpaved
6 hours ago $2.98 earnedNFL Free Agency
50.9K3 -
 18:25
18:25
Stephen Gardner
6 hours ago🔥The REAL REASON the Epstein Files are being HIDDEN | I CONFRONT Alan Dershowitz for details!
63.3K109 -
 1:58:44
1:58:44
The Quartering
9 hours agoTrump To Charge USAID Staff, Campus RIOT Erupts, Theo Von & Candace Owens, Ukraine Gets Worse!
114K72