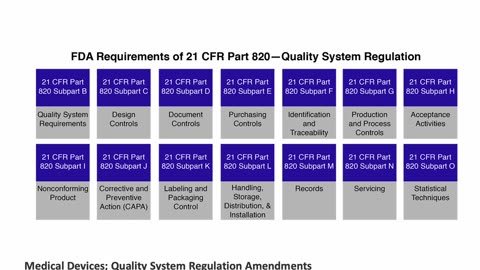
Regulatory Affairs - Applications of QSR for Medical Devices: 21 CFR Part 820 and ISO 13485 by Peivand Pirouzi, Ph.D.
Application of Quality System Regulation in Medical Device Design & Manufacturing
























Enjoy an ad-free viewing experience and other benefits